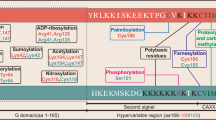
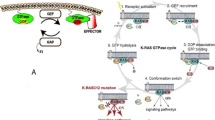
Abstract
Mutation in RAS gene is one of the most common genetic alterations, which seems to be seen in one third of human cancers. Ras, as a molecular switch, has been considered in wide variety of signaling pathways such as cell division and apoptosis. Ras proteins have a binary function to transmit different extracellular messages into intracellular signaling network. It has been proved that Ras proteins associate with different plasma membranes. Although all Ras isoforms have been found at plasma membrane, H-Ras and N-Ras are located in Golgi, and K-Ras at ER and mitochondria outer membrane. There have been a lot of efforts to inhibit Ras signaling that can be a pivotal approach to treat Ras-induced tumors. Effect of RalA and RalB on the growth of embryonal tumors, at downstream region of Ras, has been studied in a number of studies, which showed that inhibition of these signaling pathways can provide a strong therapeutic approach to cancer. Also, post translational modifications (PTMs) in proteins interfere extremely with cell signaling pathways in cells that can react to external signals. In this review, the role of Ral signaling in cancer and PTM of Ras proteins has been reviewed.

Similar content being viewed by others
References
Ahearn, I. M., Haigis, K., Bar-Sagi, D., & Philips, M. R. (2012). Regulating the regulator: Post-translational modification of RAS. Nature Reviews Molecular Cell Biology, 13(1), 39–51.
Ahearn, I., Zhou, M., & Philips, M. R. (2018). Posttranslational modifications of RAS proteins. Cold Spring Harbor Perspectives in Medicine, 8(11), a031484.
Alvarez-Moya, B., Lopez-Alcala, C., Drosten, M., Bachs, O., & Agell, N. (2010). K-Ras4B phosphorylation at Ser181 is inhibited by calmodulin and modulates K-Ras activity and function. Oncogene, 29(44), 5911–5922.
Apolloni, A., Prior, I. A., Lindsay, M., Parton, R. G., & Hancock, J. F. (2000). H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Molecular and Cellular Biology, 20(7), 2475–2487.
Aranda, E., Lopez-Pedrera, C., De La Haba-Rodriguez, R. J., & Rodriguez-Ariza, A. (2012). Nitric oxide and cancer: The emerging role of S-nitrosylation. Current Molecular Medicine, 12(1), 50–67.
Awasthi, S., Cheng, J., Singhal, S. S., Saini, M. K., Pandya, U., Pikula, S., Awasthi, Y. C., et al. (2000). Novel function of human RLIP76: ATP-dependent transport of glutathione conjugates and doxorubicin. Biochemistry, 39(31), 9327–9334.
Baker, R., Wilkerson, E. M., Sumita, K., Isom, D. G., Sasaki, A. T., Dohlman, H. G., & Campbell, S. L. (2013). Differences in the regulation of K-Ras and H-Ras isoforms by monoubiquitination. Journal of Biological Chemistry, 288(52), 36856–36862.
Ballester, R., Furth, M., & Rosen, O. (1987). Phorbol ester-and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. Journal of Biological Chemistry, 262(6), 2688–2695.
Beel, S., Kolloch, L., Apken, L. H., Jürgens, L., Bolle, A., Sudhof, N., Steinestel, K., et al. (2020). κB-Ras and Ral GTPases regulate acinar to ductal metaplasia during pancreatic adenocarcinoma development and pancreatitis. Nature Communications, 11(1), 1–16.
Bergo, M. O., Ambroziak, P., Gregory, C., George, A., Otto, J. C., Kim, E., Young, S. G., et al. (2002). Absence of the CAAX endoprotease Rce1: Effects on cell growth and transformation. Molecular and Cellular Biology, 22(1), 171–181.
Bergo, M. O., Gavino, B. J., Hong, C., Beigneux, A. P., McMahon, M., Casey, P. J., & Young, S. G. (2004). Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf. The Journal of Clinical Investigation, 113(4), 539–550.
Biondini, M., Duclos, G., Meyer-Schaller, N., Silberzan, P., Camonis, J., & Parrini, M. C. (2015). RalB regulates contractility-driven cancer dissemination upon TGFβ stimulation via the RhoGEF GEF-H1. Scientific Reports, 5(1), 1–14.
Bivona, T. G., De Castro, I. P., Ahearn, I. M., Grana, T. M., Chiu, V. K., Lockyer, P. J., Philips, M. R., et al. (2003). Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature, 424(6949), 694–698.
Bivona, T. G., Quatela, S. E., Bodemann, B. O., Ahearn, I. M., Soskis, M. J., Mor, A., Saba, S. G., et al. (2006). PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Molecular Cell, 21(4), 481–493.
Bodemann, B. O., Orvedahl, A., Cheng, T., Ram, R. R., Ou, Y.-H., Formstecher, E., Balakireva, M., et al. (2011). RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell, 144(2), 253–267.
Bodemann, B. O., & White, M. A. (2008). Ral GTPases and cancer: Linchpin support of the tumorigenic platform. Nature Reviews Cancer, 8(2), 133–140.
Caloca, M. J., Zugaza, J. L., & Bustelo, X. R. (2003). Exchange factors of the RasGRP family mediate Ras activation in the Golgi. Journal of Biological Chemistry, 278(35), 33465–33473.
Camonis, J. H., & White, M. A. (2005). Ral GTPases: Corrupting the exocyst in cancer cells. Trends in Cell Biology, 15(6), 327–332.
Cantor, S. B., Urano, T., & Feig, L. A. (1995). Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Molecular and Cellular Biology, 15(8), 4578–4584.
Cascone, I., Selimoglu, R., Ozdemir, C., Del Nery, E., Yeaman, C., White, M., & Camonis, J. (2008). Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. The EMBO Journal, 27(18), 2375–2387.
Casey, P. J., Solski, P. A., Der, C. J., & Buss, J. E. (1989). p21ras is modified by a farnesyl isoprenoid. Proceedings of the National Academy of Sciences, 86(21), 8323–8327.
Chen, C. Y., & Faller, D. V. (1995). Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene, 11(8), 1487–1498.
Chen, Q., Quan, C., Xie, B., Chen, L., Zhou, S., Toth, R., Horiuchi, H., et al. (2014). GARNL1, a major RalGAP α subunit in skeletal muscle, regulates insulin-stimulated RalA activation and GLUT4 trafficking via interaction with 14-3-3 proteins. Cellular Signalling, 26(8), 1636–1648.
Chen, X.-W., Inoue, M., Hsu, S. C., & Saltiel, A. R. (2006). RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. Journal of Biological Chemistry, 281(50), 38609–38616.
Chen, X.-W., Leto, D., Chiang, S.-H., Wang, Q., & Saltiel, A. R. (2007). Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Developmental Cell, 13(3), 391–404.
Chen, X.-W., Leto, D., Xiong, T., Yu, G., Cheng, A., Decker, S., & Saltiel, A. R. (2011). A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Molecular Biology of the Cell, 22(1), 141–152.
Chien, Y., Kim, S., Bumeister, R., Loo, Y.-M., Kwon, S. W., Johnson, C. L., Gale, M., Jr., et al. (2006). RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell, 127(1), 157–170.
Chien, Y., & White, M. A. (2003). RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Reports, 4(8), 800–806.
Chiu, V. K., Bivona, T., Hach, A., Sajous, J. B., Silletti, J., Wiener, H., Philips, M. R., et al. (2002). Ras signalling on the endoplasmic reticulum and the Golgi. Nature Cell Biology, 4(5), 343–350.
Choy, E., Chiu, V. K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Philips, M. R., et al. (1999). Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell, 98(1), 69–80.
Crespo, N. C., Ohkanda, J., Yen, T. J., Hamilton, A. D., & Sebti, S. D. M. (2001). The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. Journal of Biological Chemistry, 276(19), 16161–16167.
de Bruyn, K. M., de Rooij, J., Wolthuis, R. M., Rehmann, H., Wesenbeek, J., Cool, R. H., Bos, J. L., et al. (2000). RalGEF2, a pleckstrin homology domain containing guanine nucleotide exchange factor for Ral. Journal of Biological Chemistry, 275(38), 29761–29766.
de Gorter, D. J., Reijmers, R. M., Beuling, E. A., Naber, H. P., Kuil, A., Kersten, M. J., Spaargaren, M., et al. (2008). The small GTPase Ral mediates SDF-1–induced migration of B cells and multiple myeloma cells. Blood, the Journal of the American Society of Hematology, 111(7), 3364–3372.
Dower, N. A., Stang, S. L., Bottorff, D. A., Ebinu, J. O., Dickie, P., Ostergaard, H. L., & Stone, J. C. (2000). RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature Immunology, 1(4), 317–321.
Ebinu, J. O., Stang, S. L., Teixeira, C., Bottorff, D. A., Hooton, J., Blumberg, P. M., Stone, J. C., et al. (2000). RasGRP links T-cell receptor signaling to Ras. Blood, the Journal of the American Society of Hematology, 95(10), 3199–3203.
Ehrhardt, A., Ehrhardt, G. R., Guo, X., & Schrader, J. W. (2002). Ras and relatives—job sharing and networking keep an old family together. Experimental Hematology, 30(10), 1089–1106.
Emkey, R., Freedman, S., & Feig, L. (1991). Characterization of a GTPase-activating protein for the Ras-related Ral protein. Journal of Biological Chemistry, 266(15), 9703–9706.
Falsetti, S. C., Wang, D.-A., Peng, H., Carrico, D., Cox, A. D., Der, C. J., Sebti, S. M., et al. (2007). Geranylgeranyltransferase I inhibitors target RalB to inhibit anchorage-dependent growth and induce apoptosis and RalA to inhibit anchorage-independent growth. Molecular and Cellular Biology, 27(22), 8003–8014.
Farnsworth, C. C., Gelb, M. H., & Glomset, J. A. (1990). Identification of geranylgeranyl-modified proteins in HeLa cells. Science, 247(4940), 320–322.
Farnsworth, C. C., Wolda, S. L., Gelb, M. H., & Glomset, J. A. (1989). Human lamin B contains a farnesylated cysteine residue. Journal of Biological Chemistry, 264(34), 20422–20429.
Fernández, R. M., Ruiz-Miró, M., Dolcet, X., Aldea, M., & Garí, E. (2011). Cyclin D1 interacts and collaborates with Ral GTPases enhancing cell detachment and motility. Oncogene, 30(16), 1936–1946.
Fruman, D. A., & Rommel, C. (2014). PI3K and cancer: Lessons, challenges and opportunities. Nature Reviews Drug Discovery, 13(2), 140–156.
Fukai, S., Matern, H. T., Jagath, J. R., Scheller, R. H., & Brunger, A. T. (2003). Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. The EMBO Journal, 22(13), 3267–3278.
Gao, P., Liu, S., Yoshida, R., Shi, C., Yoshimachi, S., Sakata, N., Nakayama, H., et al. (2019). Ral GTPase activation by downregulation of RalGAP enhances oral squamous cell carcinoma progression. Journal of Dental Research, 98(9), 1011–1019.
Gentry, L. R., Martin, T. D., Reiner, D. J., & Der, C. J. (2014). Ral small GTPase signaling and oncogenesis: more than just 15 minutes of fame. Biochimica Et Biophysica Acta (BBA)-Molecular Cell Research, 1843(12), 2976–2988.
Gentry, L. R., Nishimura, A., Cox, A. D., Martin, T. D., Tsygankov, D., Nishida, M., Der, C. J., et al. (2015). Divergent roles of CAAX motif-signaled posttranslational modifications in the regulation and subcellular localization of Ral GTPases. Journal of Biological Chemistry, 290(37), 22851–22861.
Goldfinger, L. E., Ptak, C., Jeffery, E. D., Shabanowitz, J., Hunt, D. F., & Ginsberg, M. H. (2006). RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. The Journal of Cell Biology, 174(6), 877–888.
Guin, S., Ru, Y., Wynes, M. W., Mishra, R., Lu, X., Owens, C., Kern, J. A., et al. (2013). Contributions of KRAS and RAL in non–small-cell lung cancer growth and progression. Journal of Thoracic Oncology, 8(12), 1492–1501.
Győrffy, B., Stelniec-Klotz, I., Sigler, C., Kasack, K., Redmer, T., Qian, Y., & Schäfer, R. (2015). Effects of RAL signal transduction in KRAS-and BRAF-mutated cells and prognostic potential of the RAL signature in colorectal cancer. Oncotarget, 6(15), 13334.
Hahn, W. C., Counter, C. M., Lundberg, A. S., Beijersbergen, R. L., Brooks, M. W., & Weinberg, R. A. (1999). Creation of human tumour cells with defined genetic elements. Nature, 400(6743), 464–468.
Halloran, M., Parakh, S., & Atkin, J. (2013). The role of s-nitrosylation and s-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. International Journal of Cell Biology, 2013, 5.
Hamada, M., Miki, T., Iwai, S., Shimizu, H., & Yura, Y. (2011). Involvement of RhoA and RalB in geranylgeranyltransferase I inhibitor-mediated inhibition of proliferation and migration of human oral squamous cell carcinoma cells. Cancer Chemotherapy and Pharmacology, 68(3), 559–569.
Han, K., Kim, M.-H., Seeburg, D., Seo, J., Verpelli, C., Han, S., Kim, K., et al. (2009). Regulated RalBP1 binding to RalA and PSD-95 controls AMPA receptor endocytosis and LTD. PLoS Biology, 7(9), e1000187.
Hancock, J. F. (2003). Ras proteins: Different signals from different locations. Nature Reviews Molecular Cell Biology, 4(5), 373–385.
Hazelett, C. C., Sheff, D., & Yeaman, C. (2011). RalA and RalB differentially regulate development of epithelial tight junctions. Molecular Biology of the Cell, 22(24), 4787–4800.
Hazelett, C. C., & Yeaman, C. (2012). Sec5 and Exo84 mediate distinct aspects of RalA-dependent cell polarization. PLoS ONE, 7(6), e39602.
Heo, J., & Campbell, S. L. (2004). Mechanism of p21Ras S-nitrosylation and kinetics of nitric oxide-mediated guanine nucleotide exchange. Biochemistry, 43(8), 2314–2322.
Jeong, W.-J., Yoon, J., Park, J.-C., Lee, S.-H., Lee, S.-H., Kaduwal, S., Choi, K.-Y., et al. (2012). Ras stabilization through aberrant activation of Wnt/β-catenin signaling promotes intestinal tumorigenesis. Science Signaling, 5(219), ra30–ra31.
Jin, R., Junutula, J. R., Matern, H. T., Ervin, K. E., Scheller, R. H., & Brunger, A. T. (2005). Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. The EMBO Journal, 24(12), 2064–2074.
Jullien-Flores, V., Dorseuil, O., Romero, F., Letourneur, F., Saragosti, S., Berger, R., Camonis, J. H., et al. (1995). Bridging Ral GTPase to Rho pathways: RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. Journal of Biological Chemistry, 270(38), 22473–22477.
Jullien-Flores, V., Mahé, Y., Mirey, G., Leprince, C., Meunier-Bisceuil, B., Sorkin, A., & Camonis, J. H. (2000). RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: Involvement of the Ral pathway in receptor endocytosis. Journal of Cell Science, 113(16), 2837–2844.
Jura, N., Scotto-Lavino, E., Sobczyk, A., & Bar-Sagi, D. (2006). Differential modification of Ras proteins by ubiquitination. Molecular Cell, 21(5), 679–687.
Karin, M., & Hunter, T. (1995). Transcriptional control by protein phosphorylation: Signal transmission from the cell surface to the nucleus. Current Biology, 5(7), 747–757.
Kashatus, D. F. (2013). Ral GTPases in tumorigenesis: Emerging from the shadows. Experimental Cell Research, 319(15), 2337–2342.
Kawato, M., Shirakawa, R., Kondo, H., Higashi, T., Ikeda, T., Okawa, K., Horiuchi, H., et al. (2008). Regulation of platelet dense granule secretion by the Ral GTPase-exocyst pathway. Journal of Biological Chemistry, 283(1), 166–174.
Khawaja, H., Campbell, A., Roberts, J. Z., Javadi, A., O’Reilly, P., McArt, D., Bardelli, A., et al. (2020). RALB GTPase: A critical regulator of DR5 expression and TRAIL sensitivity in KRAS mutant colorectal cancer. Cell Death & Disease, 11(10), 1–18.
Kikuchi, A., Demo, S. D., Ye, Z.-H., Chen, Y.-W., & Williams, L. T. (1994). ralGDS family members interact with the effector loop of ras p21. Molecular and Cellular Biology, 14(11), 7483–7491.
Kim, E., Ambroziak, P., Otto, J. C., Taylor, B., Ashby, M., Shannon, K., Young, S. G., et al. (1999). Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras within cells. Journal of Biological Chemistry, 274(13), 8383–8390.
Kim, S.-E., Yoon, J.-Y., Jeong, W.-J., Jeon, S.-H., Park, Y., Yoon, J.-B., Choi, K.-Y., et al. (2009). H-Ras is degraded by Wnt/β-catenin signaling via β-TrCP-mediated polyubiquitylation. Journal of Cell Science, 122(6), 842–848.
Kohl, N. E., Mosser, S. D., DeSolms, S. J., Giuliani, E. A., Pompliano, D. L., Graham, S. L., Gibbs, J. B., et al. (1993). Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science, 260(5116), 1934–1937.
Lander, H. M., Hajjar, D. P., Hempstead, B. L., Mirza, U. A., Chait, B. T., Campbell, S., & Quilliam, L. A. (1997). A molecular redox switch on p21ras: Structural basis for the nitric oxide-p21ras interaction. Journal of Biological Chemistry, 272(7), 4323–4326.
Lander, H. M., Milbank, A. J., Tauras, J. M., Hajjar, D. P., Hempstead, B. L., Schwartz, G. D., Burk, S. C., et al. (1996). Redox regulation of cell signalling. Nature, 381(6581), 380–381.
Lerner, E. C., Qian, Y., Blaskovich, M. A., Fossum, R. D., Vogt, A., Sun, J., Sebti, S. M., et al. (1995). Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. Journal of Biological Chemistry, 270(45), 26802–26806.
Leto, D., Uhm, M., Williams, A., Chen, X.-W., & Saltiel, A. R. (2013). Negative regulation of the RalGAP complex by 14-3-3. Journal of Biological Chemistry, 288(13), 9272–9283.
Li, G., Han, L., Chou, T.-C., Fujita, Y., Arunachalam, L., Xu, A., Wang, L., et al. (2007). RalA and RalB function as the critical GTP sensors for GTP-dependent exocytosis. Journal of Neuroscience, 27(1), 190–202.
Lim, K.-H., Ancrile, B. B., Kashatus, D. F., & Counter, C. M. (2008). Tumour maintenance is mediated by eNOS. Nature, 452(7187), 646–649.
Lim, K.-H., Brady, D. C., Kashatus, D. F., Ancrile, B. B., Der, C. J., Cox, A. D., & Counter, C. M. (2010). Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA. Molecular and Cellular Biology, 30(2), 508–523.
Lim, K.-H., O’Hayer, K., Adam, S. J., Kendall, S. D., Campbell, P. M., Der, C. J., & Counter, C. M. (2006). Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Current Biology, 16(24), 2385–2394.
Liu, M., Sjogren, A.-K.M., Karlsson, C., Ibrahim, M. X., Andersson, K. M., Olofsson, F. J., Chen, Z., et al. (2010). Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proceedings of the National Academy of Sciences, 107(14), 6471–6476.
Ljubicic, S., Bezzi, P., Vitale, N., & Regazzi, R. (2009). The GTPase RalA regulates different steps of the secretory process in pancreatic β-cells. PLoS ONE, 4(11), e7770.
Lobell, R. B., Liu, D., Buser, C. A., Davide, J. P., DePuy, E., Hamilton, K., Motzel, S. L., et al. (2002). Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl: Protein transferase and geranylgeranyl: Protein transferase type-I. Molecular Cancer Therapeutics, 1(9), 747–758.
Lu, J., Chan, L., Fiji, H. D., Dahl, R., Kwon, O., & Tamanoi, F. (2009). In vivo antitumor effect of a novel inhibitor of protein geranylgeranyltransferase-I. Molecular Cancer Therapeutics, 8(5), 1218–1226.
Maehama, T., Tanaka, M., Nishina, H., Murakami, M., Kanaho, Y., & Hanada, K. (2008). RalA functions as an indispensable signal mediator for the nutrient-sensing system. Journal of Biological Chemistry, 283(50), 35053–35059.
Male, H., Patel, V., Jacob, M. A., Borrego-Diaz, E., Wang, K., Young, D. A., O’Brien-Ladner, A., et al. (2012). Inhibition of RalA signaling pathway in treatment of non-small cell lung cancer. Lung Cancer, 77(2), 252–259.
Marozkina, N. V., Wei, C., Yemen, S., Wallrabe, H., Nagji, A. S., Liu, L., Gaston, B., et al. (2012). S-nitrosoglutathione reductase in human lung cancer. American Journal of Respiratory Cell and Molecular Biology, 46(1), 63–70.
Martegani, E., Ceriani, M., Tisi, R., & Berruti, G. (2002). Cloning and characterization of a new Ral-GEF expressed in mouse testis. Annals of the New York Academy of Sciences, 973(1), 135–137.
Martin, T. D., Chen, X.-W., Kaplan, R. E., Saltiel, A. R., Walker, C. L., Reiner, D. J., & Der, C. J. (2014). Ral and Rheb GTPase activating proteins integrate mTOR and GTPase signaling in aging, autophagy, and tumor cell invasion. Molecular Cell, 53(2), 209–220.
Martin, T. D., & Der, C. J. (2012). Differential involvement of RalA and RalB in colorectal cancer. Small GTPases, 3(2), 126–130.
Martin, T. D., Mitin, N., Cox, A. D., Yeh, J. J., & Der, C. J. (2012). Phosphorylation by protein kinase Cα regulates RalB small GTPase protein activation, subcellular localization, and effector utilization. Journal of Biological Chemistry, 287(18), 14827–14836.
McLaughlin, S., & Aderem, A. (1995). The myristoyl-electrostatic switch: A modulator of reversible protein-membrane interactions. Trends in Biochemical Sciences, 20(7), 272–276.
Michaelson, D., Ali, W., Chiu, V. K., Bergo, M., Silletti, J., Wright, L., Philips, M., et al. (2005). Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Molecular Biology of the Cell, 16(4), 1606–1616.
Moghadam, A. R., Patrad, E., Tafsiri, E., Peng, W., Fangman, B., Pluard, T. J., Ricke, B., et al. (2017). Ral signaling pathway in health and cancer. Cancer Medicine, 6(12), 2998–3013.
Mollberg, N., Steinert, G., Aigner, M., Hamm, A., Lin, F.-J., Elbers, H., Koch, M., et al. (2012). Overexpression of RalBP1 in colorectal cancer is an independent predictor of poor survival and early tumor relapse. Cancer Biology & Therapy, 13(8), 694–700.
Monteiro, H., Costa, P., Reis, A., & Stern, A. (2015). Nitric oxide: protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomedical Journal, 38, 5.
Moskalenko, S., Henry, D. O., Rosse, C., Mirey, G., Camonis, J. H., & White, M. A. (2002). The exocyst is a Ral effector complex. Nature Cell Biology, 4(1), 66–72.
Moskalenko, S., Tong, C., Rosse, C., Mirey, G., Formstecher, E., Daviet, L., White, M. A., et al. (2003). Ral GTPases regulate exocyst assembly through dual subunit interactions. Journal of Biological Chemistry, 278(51), 51743–51748.
Nakashima, S., Morinaka, K., Koyama, S., Ikeda, M., Kishida, M., Okawa, K., Kikuchi, A., et al. (1999). Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. The EMBO Journal, 18(13), 3629–3642.
Neel, N. F., Martin, T. D., Stratford, J. K., Zand, T. P., Reiner, D. J., & Der, C. J. (2011). The RalGEF-Ral effector signaling network: The road less traveled for anti-Ras drug discovery. Genes & Cancer, 2(3), 275–287.
Neel, N. F., Rossman, K. L., Martin, T. D., Hayes, T. K., Yeh, J. J., & Der, C. J. (2012). The RalB small GTPase mediates formation of invadopodia through a GTPase-activating protein-independent function of the RalBP1/RLIP76 effector. Molecular and Cellular Biology, 32(8), 1374–1386.
Neyraud, V., Aushev, V. N., Hatzoglou, A., Meunier, B., Cascone, I., & Camonis, J. (2012). RalA and RalB proteins are ubiquitinated GTPases, and ubiquitinated RalA increases lipid raft exposure at the plasma membrane. Journal of Biological Chemistry, 287(35), 29397–29405.
Nishimura, A., & Linder, M. E. (2013). Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding. Molecular and Cellular Biology, 33(7), 1417–1429.
Oeckinghaus, A., Postler, T. S., Rao, P., Schmitt, H., Schmitt, V., Grinberg-Bleyer, Y., Ghosh, S., et al. (2014). κB-Ras proteins regulate both NF-κB-dependent inflammation and Ral-dependent proliferation. Cell Reports, 8(6), 1793–1807.
Oxford, G., Owens, C. R., Titus, B. J., Foreman, T. L., Herlevsen, M. C., Smith, S. C., & Theodorescu, D. (2005). RalA and RalB: Antagonistic relatives in cancer cell migration. Cancer Research, 65(16), 7111–7120.
Papini, D., Langemeyer, L., Abad, M. A., Kerr, A., Samejima, I., Eyers, P. A., Earnshaw, W. C., et al. (2015). TD-60 links RalA GTPase function to the CPC in mitosis. Nature Communications, 6(1), 1–12.
Park, S.-H., & Weinberg, R. A. (1995). A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene, 11(11), 2349–2355.
Peterson, S. N., Trabalzini, L., Brtva, T. R., Fischer, T., Altschuler, D. L., Martelli, P., White, G. C., II., et al. (1996). Identification of a novel RalGDS-related protein as a candidate effector for Ras and Rap1. Journal of Biological Chemistry, 271(47), 29903–29908.
Rebhun, J. F., Chen, H., & Quilliam, L. A. (2000). Identification and characterization of a new family of guanine nucleotide exchange factors for the ras-related GTPase Ral. Journal of Biological Chemistry, 275(18), 13406–13410.
Resh, M. D. (2013). Covalent lipid modifications of proteins. Current Biology, 23(10), R431–R435.
Rossé, C., L’Hoste, S., Offner, N., Picard, A., & Camonis, J. (2003). RLIP, an effector of the Ral GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. Journal of Biological Chemistry, 278(33), 30597–30604.
Sablina, A. A., Chen, W., Arroyo, J. D., Corral, L., Hector, M., Bulmer, S. E., Hahn, W. C., et al. (2007). The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell, 129(5), 969–982.
Sasaki, A. T., Carracedo, A., Locasale, J. W., Anastasiou, D., Takeuchi, K., Kahoud, E. R., Cantley, L. C., et al. (2011). Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Science Signaling, 4(163), ra14–ra15.
Seguin, L., Kato, S., Franovic, A., Camargo, M. F., Lesperance, J., Elliott, K. C., Husain, H., et al. (2014). An integrin β 3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nature Cell Biology, 16(5), 457–468.
Seibold, M., Stühmer, T., Kremer, N., Mottok, A., Scholz, C.-J., Schlosser, A., Barrio, S., et al. (2020). RAL GTPases mediate multiple myeloma cell survival and are activated independently of oncogenic RAS. Haematologica, 105(9), 2316.
Shao, H., & Andres, D. A. (2000). A novel RalGEF-like protein, RGL3, as a candidate effector for rit and Ras. Journal of Biological Chemistry, 275(35), 26914–26924.
Shipitsin, M., & Feig, L. A. (2004). RalA but not RalB enhances polarized delivery of membrane proteins to the basolateral surface of epithelial cells. Molecular and Cellular Biology, 24(13), 5746–5756.
Shirakawa, R., Fukai, S., Kawato, M., Higashi, T., Kondo, H., Ikeda, T., Kimura, T., et al. (2009). Tuberous sclerosis tumor suppressor complex-like complexes act as GTPase-activating proteins for Ral GTPases. Journal of Biological Chemistry, 284(32), 21580–21588.
Shirakawa, R., & Horiuchi, H. (2015). Ral GTPases: Crucial mediators of exocytosis and tumourigenesis. The Journal of Biochemistry, 157(5), 285–299.
Shukla, S., Allam, U. S., Ahsan, A., Chen, G., Krishnamurthy, P. M., Marsh, K., Schipper, M., et al. (2014). KRAS protein stability is regulated through SMURF2: UBCH5 complex-mediated β-TrCP1 degradation. Neoplasia, 16(2), 115-W115.
Sidhu, R. S., Clough, R. R., & Bhullar, R. P. (2003). Ca2+/calmodulin binds and dissociates K-RasB from membrane. Biochemical and Biophysical Research Communications, 304(4), 655–660.
Siegel-Lakhai, W., Crul, M., Zhang, S., Sparidans, R., Pluim, D., Howes, A., Schellens, J., et al. (2005). Phase I and pharmacological study of the farnesyltransferase inhibitor tipifarnib (Zarnestra®, R115777) in combination with gemcitabine and cisplatin in patients with advanced solid tumours. British Journal of Cancer, 93(11), 1222–1229.
Simicek, M., Lievens, S., Laga, M., Guzenko, D., Aushev, V. N., Kalev, P., Tavernier, J., et al. (2013). The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response. Nature Cell Biology, 15(10), 1220–1230.
Sjogren, A.-K.M., Andersson, K. M., Liu, M., Cutts, B. A., Karlsson, C., Wahlstrom, A. M., Tarkowski, A., et al. (2007). GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS–induced lung cancer. The Journal of Clinical Investigation, 117(5), 1294–1304.
Song, X., Hua, L., Xu, Y., Fang, Z., Wang, Y., Gao, J., Yu, R., et al. (2015). Involvement of RalB in the effect of geranylgeranyltransferase I on glioma cell migration and invasion. Clinical and Translational Oncology, 17(6), 477–485.
Sparano, J. A., Moulder, S., Kazi, A., Coppola, D., Negassa, A., Vahdat, L., Munster, P., et al. (2009). Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clinical Cancer Research, 15(8), 2942–2948.
Sparano, J. A., Moulder, S., Kazi, A., Vahdat, L., Li, T., Pellegrino, C., Hoschander, S., et al. (2006). Targeted inhibition of farnesyltransferase in locally advanced breast cancer: A phase I and II trial of tipifarnib plus dose-dense doxorubicin and cyclophosphamide. Journal of Clinical Oncology, 24(19), 3013–3018.
Sugihara, K., Asano, S., Tanaka, K., Iwamatsu, A., Okawa, K., & Ohta, Y. (2002). The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nature Cell Biology, 4(1), 73–78.
Sun, J., Qian, Y., Hamilton, A. D., & Sebti, S. M. (1995). Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-Ras mutation and p53 deletion. Cancer Research, 55(19), 4243–4247.
Switzer, C. H., Cheng, R. Y., Ridnour, L. A., Glynn, S. A., Ambs, S., & Wink, D. A. (2012). Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Research, 14(5), 1–13.
Tang, C.-H., Wei, W., & Liu, L. (2012). Regulation of DNA repair by S-nitrosylation. Biochimica Et Biophysica Acta (BBA)-General Subjects, 1820(6), 730–735.
Urano, T., Emkey, R., & Feig, L. A. (1996). Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. The EMBO Journal, 15(4), 810–816.
Vigil, D., Martin, T. D., Williams, F., Yeh, J. J., Campbell, S. L., & Der, C. J. (2010). Aberrant overexpression of the Rgl2 Ral small GTPase-specific guanine nucleotide exchange factor promotes pancreatic cancer growth through Ral-dependent and Ral-independent mechanisms. Journal of Biological Chemistry, 285(45), 34729–34740.
Wahlstrom, A. M., Cutts, B. A., Liu, M., Lindskog, A., Karlsson, C., Sjogren, A.-K.M., Bergo, M. O., et al. (2008). Inactivating Icmt ameliorates K-RAS–induced myeloproliferative disease. Blood, the Journal of the American Society of Hematology, 112(4), 1357–1365.
Wang, C., Yuan, P., Xu, B., Yuan, L., Yang, H., & Liu, X. (2015). RLIP76 expression as a prognostic marker of breast cancer. European Review for Medical and Pharmacological Sciences, 19(11), 2105–2111.
Wang, H., Owens, C., Chandra, N., Conaway, M. R., Brautigan, D. L., & Theodorescu, D. (2010). Phosphorylation of RalB is important for bladder cancer cell growth and metastasis. Cancer Research, 70(21), 8760–8769.
Wang, M., Hossain, M., Tan, W., Coolman, B., Zhou, J., Liu, S., & Casey, P. (2010). Inhibition of isoprenylcysteine carboxylmethyltransferase induces autophagic-dependent apoptosis and impairs tumor growth. Oncogene, 29(35), 4959–4970.
Wang, Q., Wang, J.-Y., Zhang, X.-P., Lv, Z.-W., Fu, D., Lu, Y.-C., Chen, J.-X., et al. (2013). RLIP76 is overexpressed in human glioblastomas and is required for proliferation, tumorigenesis and suppression of apoptosis. Carcinogenesis, 34(4), 916–926.
White, M. A., Vale, T., Camonis, J. H., Schaefer, E., & Wigler, M. H. (1996). A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. Journal of Biological Chemistry, 271(28), 16439–16442.
Whyte, D. B., Kirschmeier, P., Hockenberry, T. N., Nunez-Oliva, I., James, L., Catino, J. J., Pai, J.-K., et al. (1997). K-and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. Journal of Biological Chemistry, 272(22), 14459–14464.
Williams, J. G., Pappu, K., & Campbell, S. L. (2003). Structural and biochemical studies of p21Ras S-nitrosylation and nitric oxide-mediated guanine nucleotide exchange. Proceedings of the National Academy of Sciences, 100(11), 6376–6381.
Willumsen, B. M., Christensen, A., Hubbert, N. L., Papageorge, A. G., & Lowy, D. R. (1984). The p21 ras C-terminus is required for transformation and membrane association. Nature, 310(5978), 583–586.
Winter-Vann, A. M., Kamen, B. A., Bergo, M. O., Young, S. G., Melnyk, S., James, S. J., & Casey, P. J. (2003). Targeting Ras signaling through inhibition of carboxyl methylation: An unexpected property of methotrexate. Proceedings of the National Academy of Sciences, 100(11), 6529–6534.
Wolda, S. L., & Glomset, J. (1988). Evidence for modification of lamin B by a product of mevalonic acid. Journal of Biological Chemistry, 263(13), 5997–6000.
Wu, Z., Owens, C., Chandra, N., Popovic, K., Conaway, M., & Theodorescu, D. (2010). RalBP1 is necessary for metastasis of human cancer cell lines. Neoplasia, 12(12), 1003–1012.
Xia, S., Forman, L. W., & Faller, D. V. (2007). Protein kinase Cδ is required for survival of cells expressing activated p21RAS. Journal of Biological Chemistry, 282(18), 13199–13210.
Xu, L., Lubkov, V., Taylor, L. J., & Bar-Sagi, D. (2010). Feedback regulation of Ras signaling by Rabex-5-mediated ubiquitination. Current Biology, 20(15), 1372–1377.
Yan, C., Liu, D., Li, L., Wempe, M. F., Guin, S., Khanna, M., Wysoczynski, C. L., et al. (2014). Discovery and characterization of small molecules that target the GTPase Ral. Nature, 515(7527), 443–447.
Yan, C., & Theodorescu, D. (2018). RAL GTPases: Biology and potential as therapeutic targets in cancer. Pharmacological Reviews, 70(1), 1–11.
Yan, H., Jahanshahi, M., Horvath, E. A., Liu, H.-Y., & Pfleger, C. M. (2010). Rabex-5 ubiquitin ligase activity restricts Ras signaling to establish pathway homeostasis in Drosophila. Current Biology, 20(15), 1378–1382.
Yang, M. H., Laurent, G., Bause, A. S., Spang, R., German, N., Haigis, M. C., & Haigis, K. M. (2013). HDAC6 and SIRT2 regulate the acetylation state and oncogenic activity of mutant K-RAS. Molecular Cancer Research, 11(9), 1072–1077.
Yang, M. H., Nickerson, S., Kim, E. T., Liot, C., Laurent, G., Spang, R., Bar-Sagi, D., et al. (2012). Regulation of RAS oncogenicity by acetylation. Proceedings of the National Academy of Sciences, 109(27), 10843–10848.
Yang, Q., Lang, C., Wu, Z., Dai, Y., He, S., Guo, W., Peng, X., et al. (2019). MAZ promotes prostate cancer bone metastasis through transcriptionally activating the KRas-dependent RalGEFs pathway. Journal of Experimental & Clinical Cancer Research, 38(1), 1–17.
Zago, G., Veith, I., Singh, M. K., Fuhrmann, L., De Beco, S., Remorino, A., Brandon, N., et al. (2018). RalB directly triggers invasion downstream Ras by mobilizing the Wave complex. eLife, 7, e4074.
Zhu, Z., Aref, A. R., Cohoon, T. J., Barbie, T. U., Imamura, Y., Yang, S., Thai, T. C., et al. (2014). Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discovery, 4(4), 452–465.
Zinatizadeh, M. R., Miri, S. R., Zarandi, P. K., Chalbatani, G. M., Raposo, C., Mirzaei, H. R., Mahmoodzadeh, H., et al. (2021). The Hippo Tumor Suppressor Pathway (YAP/TAZ/TEAD/MST/LATS) and EGFR-RAS-RAF-MEK in cancer metastasis. Genes Dis, 8(1), 48–60. https://doi.org/10.1016/j.gendis.2019.11.003
Zinatizadeh, M. R., Momeni, S. A., Zarandi, P. K., Chalbatani, G. M., Dana, H., Mirzaei, H. R., Miri, S. R., et al. (2019). The role and function of Ras-association domain family in cancer: a review. Genes & Diseases, 6(4), 378–384.
Zipfel, P. A., Brady, D. C., Kashatus, D. F., Ancrile, B. D., Tyler, D. S., & Counter, C. M. (2010). Ral activation promotes melanomagenesis. Oncogene, 29(34), 4859–4864.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Zinatizadeh, M.R., Zarandi, P.K., Keshavarz-Fathi, M. et al. The role of ral signaling and post translational modifications (PTMs) of Ras in cancer. GENOME INSTAB. DIS. 3, 22–32 (2022). https://doi.org/10.1007/s42764-022-00059-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42764-022-00059-0