Abstract
The equilibrium and kinetic characteristics of rare earths adsorption process using the strong acid macro reticular resin Dowex 50X8 from El-Erediya chloride liqueurs have been determined. The adsorption parameters including pH, contact time, temperature, resin to aqueous ratio and rare earths initial concentration in aqueous have been optimized. The practical adsorption capacity of rare earths upon the resin under the optimum conditions has been found to attain 80 mg/g which matches with Langmuir theoretical capacity. The physical parameters including the adsorption kinetics, the isotherm models and the thermodynamic data have also been determined. The adsorption process has been found to agree with both the pseudo second order reaction, the Langmuir isotherm and confirms with the concept of the matrix diffusion controls. A successful preconcentration process for the rare earths content was done with a preconcentration factor of about 42.33. Considerable rare earths cake was precipitated from the chloride eluate solutions using oxalic acid.
Similar content being viewed by others
1 Introduction
An increase in demand for rare earth elements (REEs) has been observed in the recent years because of their unique properties and various applications. Presently, six major countries reportedly produced REEs, namely China, Australia, United States, Russia, Thailand, and Malaysia [1]. China has become the largest world supplier which reportedly produces more than 95% of total rare earth oxide (REO). In line with the recent drastic advancement in technology, REEs are becoming increasingly important sources of advanced science materials such as electronic, environmental, optical, magnetic and catalytic technologies. It has to be noted that these elements have now become more important for highly technological applications and most of the industries reportedly use REEs more than platinum group metals [2] and they are notably used more than gold [3].
The cation exchange resins are the earliest applications for the group separation of rare earth elements. Commonly used strong cation exchange resins include Dowex AG 50 W-X8 [4], Dowex AG 50WX12 [5], Bio Rad AG 50-X8 [6], Ostion LGKS 0800, sulphonated polystyrene and bonded-phase silica [7] and Amberlite IR-120 [8].
El-Erediya mineralization consists of mineralizations structurally controlled and associated with jasperoid veins that are hosted by a granitic pluton [9]. This granite exhibits extensive alteration, including silicification, argillization, sericitization, chloritization, carbonatization, and hematization [10]. Primary and secondary uranium minerals uranium mineral are present in large quantities in the form of pitchblende. Pyrochlore group minerals typically contain significant amounts of REE, U and Th. These minerals are accompanied by pyrite, galena, magnetite–titanomagnetite, ilmenite, hematite, betafite, rutile, muscovite, titanite, fluorite, zircon, monazite, apatite, and tourmaline. Zircon, monazite, calcite, apatite, garnet and fluorite are presented as accessory minerals [11].
This work is concerned with the pre-concentration of the rare earths content from El-Erediya effluent solutions which collected after the uranium recovery processes, extraction of REEs from chloride liquors using cation exchange resin Dowex 50X8 and determining the equilibrium and the kinetic characteristics as well as the interesting thermodynamic data of the strong resin. To realize the objectives of this work, the various parameters of REE (III) adsorption upon the studied resin have been experimentally optimized as a pre-requisite for the determination of the relevant physical characteristics.
2 Experimental
2.1 Rare earths precipitation from sulfate effluent solution
The experimental work was performed upon an effluent solutions collected from several hydrometallurgical treatments for El-Erediya mineralization which located in the Eastern desert, Egypt. These treatments were carried out to recovery the uranium content from the sulfate leach liquors of El-Erediya mineralization and the rare earths content was remained dissolved in the desired effluents. The sulfate effluent solution of 65 mg REEs/L was then subjected to rare earths precipitation study using sodium hydroxide 50% in pH range varied from 3.4 to 9.5; this was followed by washing and drying steps.
2.2 Preparation of the chloride liquors
The hydroxide precipitates were dissolved using hydrochloric acid 30% and diluted with distilled water to perform a chloride liquors of pH 1.0. The accomplished chloride liquor was considered the main liquor in the subsequent extraction reactions for the rare earths. Complete analysis for the chloride liquors was carried out using Inductively Coupled Plasma optical emission spectrometry ICP-OES (Teledyne Leeman, Hudson, New Hampshire 03051USA) in order to determine its contents.
2.3 Resin specification and pretreatment
A strong acidic cation exchange resin Dowex 50X8 (Dow Chemical Company, Saint Louis, MO 63103, USA), which used in the present work, is a macro reticular cation exchange resin. A macro porous network resin Dowex 50X8 differs completely from the conventional gel type resins. This type of resin provides an outstanding osmotic and physical stability as well as excellent kinetics. The physical and the chemical characteristics of Dowex 50X8 resin was listed in Table 1.
Resin pretreatment was carried out before the extraction stage; this was carried out by washing the resin with distilled water and followed by diluted hydrochloric acid for several times.
2.4 Adsorption experimental procedure
The batch procedure was performed to optimize the basic equilibrium conditions for REEs adsorption such as pH, contact time, temperature and resin to liquid ratio. In these experiments, 10 ml of 300 mg REEs/L aqueous solution was stirred with 0.1 g dry resin at 200 rpm in a 50 ml conical flask using a magnetic stirrer of Fisher Scientific model. After each experiment, the two phases were decanted and the clear aqueous solution was screened and analyzed against the total REEs concentration.
Total rare earths was determined by Arsenazo III (Sigma-Aldrich, Saint Louis, MO 63103, USA) where the absorbance of its complex was measured at the wavelength [13] by using UV-spectrophotometer (SP-8001 model, Metretech Inc., Nankang, Taipei, Taiwan).
The amount of REEs adsorption qe (mg/g) was calculated from the difference of REEs concentration in the aqueous solution before and after adsorption at the equilibrium time t according to Eq. (1):
where Co and Ce are the initial and equilibrium concentrations of REE (III) in the solution (mol L−1), V is the volume of solution (L), m is the weight of the resin (g). The amount of REE (III) adsorbed onto the resin (q, mg/g (and the uptake percent (REEs %) were determined using Eq. (2). The adsorption parameters, rate and the order of reaction were calculated from experimental results by graphing using Microsoft office 2010 program.
2.5 Rare earths elution and precipitation processes
Elution process for rare earths from the loaded resin was carried out using hydrochloric acid as an eluate. The rare earths content was precipitated from eluate solution using oxalic acid addition. The rare earths oxalate precipitate was decanted, washed, ignited and analyzed using x-ray fluorescence technique (JEol-JSX-3222, Peabody, MA 01960, USA) to identify the purity of the rare earth produced cake.
3 Results and discussion
3.1 Chloride liquors characterizations
Complete dissolution for the pre-concentrated hydroxide precipitates was attained using th e hydrochloride acid. The chemical composition of the El-Erediya chloride liquors was illustrated in Table 2. From these results it was noticed that the rare earths concentration was enhanced from 60 mg/L in the sulfate liquors to about 300 mg/L in the chloride liquors. This enhancement in the rare earths concentration Led to an improvement in the extraction process.
3.2 Optimization of REEs adsorption factors
3.2.1 Effect of pH
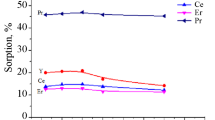
The effect of pH of the chloride liquor was studied in a range varied from 0.25 to 2.0 using fixed conditions of 0.1 g resin, 10 ml aqueous solution assaying 300 mg REEs/L and 2 h contact time at room temperature. From the obtained results showed in Fig. 1, the adsorption efficiency was increased from 4.3 to 91.4% by increasing the pH from 0.25 to 1.25 which considered the preferable pH value. The variation in the pH of the feed solution causes a variation in the kind of rare earths ions which has great effects on the interaction between the resin and the rare earths ions species presented in the leach liqueur.
3.2.2 Effect of contact time
In order to determine the equilibrium time for the REEs adsorption, a range from 2.5 to 180 min was studied as a contact time under fixed conditions of 0.1 g resin, 10 ml aqueous solution assaying 300 mg REEs/L and pH of 1.25 at room temperature. From the obtained results plotted in Fig. 1, it was indicated that the adsorption efficiency was gradually increased to reach maximum extraction by using 75 min as a contact time and remains constant thereafter. Hence, the adsorption equilibrium time of 75 min was convenient.
3.2.3 Effect of extraction temperature
The effect of temperature on the REE adsorption efficiency by Dowex 50X8 resin from chloride aqueous solution was studied within the temperature range from 25 to 55 °C under fixed conditions of 0.1 g resin, 10 ml aqueous solution assaying 300 mg REEs/L, 75 min contact time and pH of 1.25. From the results represented in Fig. 1, it was clear that the temperature has a bad effect on the adsorption process; therefore conducting the REEs extraction process at room temperature was preferred. The reduction in the REE adsorption efficiency by increasing the temperature might be due to decreasing the surface activity that leads to the decrease in the thickness of the boundary layer which increases the tendency of REEs to escape to the solution phase after extraction.
3.2.4 Effect of initial rare earth elements concentration
The effect of the initial REEs (III) concentration on the adsorption efficiency by Dowex 50X8 resin was studied in the range from 150 to 1200 mg REEs/L under fixed conditions of 0.1 g resin, 10 ml aqueous solution assaying 300 mg REEs/L, 75 min contact time and pH of 1.25 at room temperature. From the obtained data in Fig. 1, it noticed that the REEs uptake (mg/g) was sharply enhanced by increasing the REEs concentration from 150 to 300 mg/L and remains constant thereafter. Therefore, it can be ascertained that the maximum loading capacity of REEs upon Dowex 50X8 resin is 80 mg/g.
3.2.5 Effect of resin to aqueous ratio
The effect of solid resin to aqueous liquor ratio (R:A) was studied using a range from 1:100 to 1:500 (R:A) under fixed conditions of aqueous solution assaying 300 mg REEs/L, 75 min contact time and pH of 1.25 at room temperature. From the results plotted in Fig. 1, the REEs uptake was gradually enhanced by decreasing the R:A ratio from 1:100 to 1:400 to reach 80 mg REEs/L which considered the more favorable ratio.
3.3 Rare earths adsorption kinetics
In order to investigate the kinetic characteristics concerning the mechanism of rare earths adsorption by Dowex 50X8 resin and the potential rate controlling steps, several dynamic equations were applied upon the solid–liquid extraction system such as Lagergren equation.
3.3.1 Pseudo-first order and pseudo-second order models
In order to determine the order of adsorption reaction, the experimental data obtained from batch experiments of the adsorption temperature factor were evaluated using Lagergren equation for pseudo-first order model Eq. (3) or Lagergren equation for pseudo-second order model Eq. (4) to determine the rate of the adsorption interactions [14].
where qe and qt are the values of the amount REEs adsorbed per unit mass (mg g−1) at equilibrium and at any time t, respectively and kl, K2 were the first order rate constant and the second order rate constant (min−1) respectively. The Kl values could be obtained by plotting log (qe − qt) versus t for sorption of REEs at different temperatures and K2 values by plotting of t/qt versus t for sorption of REEs at different temperatures. The result of REEs adsorption mechanism by Dowex 50X8 according to pseudo-first order and pseudo-second order models were graphically plotted in Fig. 2. The values of first order rate constant and the second order rate constant (K1, K2) and correlation coefficient (R2) obtained from these plots were listed in the Table 3.
From the obtained data, the plotting of t/qt versus t for REEs adsorption model showed straight lines with good linearity with correlation coefficients closer to unity. Thus, the interaction of REEs with the resin sorbent followed second order kinetics and the values of K2 indicate that the rate of the process decreases with temperature.
3.4 Adsorption isotherm models
According to the previous mentioned isotherm models, the REEs adsorption on Dowex 50X8 resin has been described by applying the most widely used Langmuir [15] and Freundlich [16] isotherm models. The equilibrium studies are generally used to elucidate the sorption process and provide an understanding of the sorption mechanism. The Langmuir and Freundlich are most familiar, whereas, in this study, these sorption models were utilized to assess their correlation with the experimental data. The Langmuir model assumes that the maximum adsorption occurs in the saturated monolayer of adsorbate molecules on the adsorbent surface, and the energy of adsorption is constant, as well as there is no transmigration of adsorbate on the plane of the surface. The metal ions can be adsorbed from their solutions onto the surface of a solid support by several mechanisms. On the other hand, the Freundlich isotherm model is based on the assumption that adsorption occurs on a heterogeneous surface. It proposes that the adsorption occurs with a heterogeneous distribution of active sites, accompanied by interactions between adsorbed molecules.
3.4.1 Langmuir adsorption model
According to Langmuir isotherm model, the adsorption process was considered as a chemical phenomenon with the formation of energetically mono layer and a maximum adsorption capacity qmax (mg/g) calculated from Eq. (5).
where KL is a constant of the adsorption equilibrium (L/mg), qmax is the saturated monolayer adsorption capacity (mg/g) while qe and Ce are the REEs uptake capacity (mg/g) of adsorbent and the residual REEs concentration (mg/L) at equilibrium. A linearized plot with straight line was obtained by plotting of 1/qe against 1/Ce as shown in Fig. 3. The correlation coefficient of the straight line is found to be 0.951 which indicates good linear relationship, as well as the qmax and KL obtained from the intercept and the slope of Langmuir plot line were found to be 85.47 mg/g and 0.0201 l/mg respectively. Therefore, it can be concluded that the working adsorption system obeys Langmuir adsorption model and the model is suitable for the description of the adsorption equilibrium of REEs onto Dowex 50X8.
The essential characteristics of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor, RL, which is used to predict if an adsorption system is favorable or not. The separation factor, RL, is given by Eq. (6). The results of the Langmuir isotherm constants were given in Table 3.
where Co is the initial REE (III) concentration (mg/L) and KL is the Langmuir adsorption constant (L/mg). The calculated RL value for REEs adsorption of 300 mg REEs/L concentration is 0.141 which in the range between 0.0 and 1.0, this indicates that the adsorption of REE (III) on Dowex 50X8 is favorable.
3.4.2 Freundlich adsorption model
The empirical Freundlich equation, which based on the adsorption on a heterogeneous surface, is represented in Eq. (7). KF and n are the Freundlich constants which represent the adsorption capacity and the adsorption intensity respectively.
KF and 1/n can be determined from the intercept and the slope of linear plot of Log qe against Log Ce as shown in Fig. 4.
The values of the constants Kf and n were found to be 2.827 mg/g and 1.421, respectively. The value of n (1 < n > 10) indicates the adsorption of REE (III) ions onto Dowex 50X8 resin is favorable. The correlation coefficient for the Freundlich plot was found to be 0.979 indicating that the experimental data obey the Freundlich isotherm model. The results of the Freundlich isotherm constants are given in Table 4.
Finally, to investigate the best-fitting isotherm model, the results indicate that the saturated monolayer adsorption capacity qmax is equal to about 85 which closed to the practically maximum saturation capacity of the working cation exchange resin. So, the Langmuir isotherm is more favorable than Freundlich. Therefore, it can be concluded that the working adsorption system obeys Langmuir adsorption model, the studied resin is homogeneous in the liquid phase and suitable for the description of the adsorption equilibrium of REEs onto Dowex 50X8.
3.4.3 Homogeneous diffusion model (HDM)
Homogeneous Particle Diffusion Model (HPDM) is the kinetic model which is widely used to describe the adsorption process on the ion exchange resin [17]. The extraction process is carried out by diffusion of metal ions from the aqueous solution and H+ ions from the resin. The rare earths ions in the solution phase diffuse across the liquid film surrounding the ion exchange resin particle, transfer across the solution particle interface, diffuse into the bulk of the ion exchange resin particle and possibly interact with ion exchange reactive group. The film diffusion mode (FD) and matrix diffusion mode (MD) are given respectively, by Eqs. (8) and (9) which can be used to describe the rate of the reaction [18, 19]:
where X is the fractional attainment of equilibrium (X = qt/qe) (assuming a constant value of the film thickness δ). The kinetic parameters for sorption of REEs on Dowex 50X8 according to HPDM were estimated in Fig. 5. The KFD values indicate that the diffusion coefficient in the liquid film (FMD) increases with the temperature. From the linear plot of function—ln (1 − X) versus time t at different temperatures by using Eq. (8) as shown in Fig. 5a, it is clear that the straight lines passing through the origin obtained for this relation is supported by a correlation coefficient (R2) obtained around 0.80. Therefore, it is not satisfactory to explain the reaction by a film diffusion mode (FD) and the data did not fit at different temperatures.
On the other hand, the diffusion coefficients in matrix diffusion (DMD) can be estimated by using Eq. (9). The plots of function—ln (1 − X2) versus time t at different temperatures was shown in Fig. 5b. The straight lines obtained passing thought the origin satisfactory represent the data based on the higher correlation coefficients (R2) of 0.98.
Further investigations upon rare earths adsorption process on Dowex 50X8 resin using the Homogeneous Particle Diffusion Model were conducted using a stirring speed range from 100 to 250 rpm at room temperature. By plotting—Ln (1 − X) or − Ln (1 − X2) versus time t at different stirring speeds, the corresponding Film diffusion mode and the matrix diffusion mode plots for REEs sorption on Dowex 50X8 were graphed in Fig. 6.
The correlation coefficient (R2) for the straight lines passing through the origin as shown in the Film diffusion mode plot was around 0.90. Therefore, it is not satisfactory to explain the adsorption reaction and the data did not fit at different stirring speed. On the other hand, the higher correlation coefficients (around 0.99) for the straight lines obtained in matrix diffusion mode plot are created. So, the rare earths adsorption process on Dowex 50X8 resin is carried out through a matrix diffusion controlling process.
3.5 Thermodynamic of adsorption process
The thermodynamic parameters of the studied adsorption process have been determined for REEs adsorption upon Dowex 50X8 by using the following Vanʼt Hoff Eq. (10).
where Kd (ml/g), ΔH (KJ/mol), ΔS (J/mol K), T (Kelvin) and R (J/mol K) are the distribution coefficient, the enthalpy, the entropy, the temperature in Kelvin and the molar gas constant respectively [20, 21]. The distribution coefficient (Kd) of rare earths between the aqueous bulk phase and the solid phase was calculated from the following Eq. (11):
The plotting of Log Kd against 1000/T for REEs adsorption is shown in Fig. 7. The values of ΔH and ΔS were obtained from the slope and intercept of the plot while the Gibbs free energy, ΔG (KJ/mol), is calculated from the following Eq. (12).
The calculated values of the thermodynamic parameters for rare earths adsorption on Dowex 50X8 are given in Table 5. Negative value of ΔH shows that REEs adsorption has an exothermic nature. In addition, the negative ΔS parameter suggests decreasing the system randomness at the solid–liquid interface during the adsorption process. The negative value of ΔG indicates that the adsorption reaction is spontaneous and favorable at the room temperature.
3.6 Characterization of Dowex 50X8
The SEM images (Scanning electron micrograph model JEOL-JSM-5600LV) of the Dowex 50X8 resin before and after REE (III) adsorption are shown in Fig. 8a, b, respectively. The SEM images were clearly shown the difference between the surfaces of the Dowex 50 resin. Although a good uniformity and smooth surface observed in the conventional resin but the surface after REE (III) adsorption was observed a bright spherical spots on the resin beads. As could be seen from the results, a visible change of the surface morphology in the REE (III) adsorbed resin demonstrated that the sorption of REE (III) ions were taken place onto the Dowex 50X8 resin.
3.7 Rare earths elution and precipitation processes
Elution process for the rare earths from the loaded Dowex 50X8 resin was carried using hydrochloric acid as an eluate under the conditions of 4.0 mol/L hydrochloric acid, 120 min contact time, 6/1 eluate/resin ratio and stirring speed 250 rpm at ambient temperature. Therefore, the elution efficiency for rare earths from the saturated resin under these conditions reached 93%.
Several developments and tendencies on the separation and pre-concentration techniques for REEs were compared using different ion exchange resins as shown in Table 6. From the obtained results in this study, Dowex 50X8 resin was succeeded in the extraction and pre-concentration of total rare earths from a chloride feed solutions of about 300 mg REEs/L. Stripping chloride solutions of 12,400 mg/L were gained through the stripping stage of the saturated resin of 80 mg REEs/g. This means a successful preconcentration process for the rare earths content was done with a preconcentration factor (PF) of about 42.33. For example, PF of Dowex 50X8 resin for rare earths was higher than using 2,6-diacetylpyridine functionalized Amberlite XAD-4(PF = 5) and less than by using Ion-imprinted polymers; template: Sc-8-hydroxyquino-line complex (PF 60).
The properly collected eluates fractions were subjected to selective precipitation for the total rare earths constituents using oxalic acid at pH 1.0. The precipitated cake was decanted, filtered, washed by 1% oxalic acid, ignited at 850 °C and finally analyzed against its constituents using by X-ray fluorescence technique to identify the purity of the final cake. The analytical results which indicated in Fig. 9 proved the presence of considerable contents of rare earths with a presence of minor undesirable gangues.
4 Summary and conclusions
The batch tests which performed to optimize the REEs adsorption by Dowex 50X8 resin indicated that 80 mg REEs/g resin maximum saturation capacity was attained by adjusting the pH at 1.25 for 75 min contact time and with resin to liquor ratio of 1/400 at ambient temperature.
By applying Lagergren equation, the sorption reaction can be approximated more favorably by the pseudo second order sorption as the predominate mechanism and the values of k2 “second order rate constant” indicate that the rate of the process decreases with temperature.
By analysis the data of adsorption of REEs on Dowex 50X8 according to homogeneous diffusion model, the adsorption confirmed the concept of the matrix diffusion control of the process.
Both Langmuir and Freundlich isotherm models are suitable for the description of the adsorption equilibrium of REEs onto Dowex 50X8, The calculated dimensionless constant separation factor “RL” value is too small which indicates that the adsorption of REEs on Dowex 50X8 is favorable.
From the thermodynamic parameters, a negative value of ΔH shows that REEs adsorption is of an exothermic nature and the negative ΔS parameter suggests decreasing the system randomness at the solid–liquid interface during the adsorption process.
A considerable rare earths hydroxide cake was produced from the chloride eluate solutions after precipitation using oxalic acid.
References
U.S. Geological Survey, Mineral Commodity Summaries (2018) https://minerals.usgs.gov/minerals/pubs/mcs/2016/mcs2016.pdf Accessed 12 Jan 2018
Max-Hansen M (2014) Modeling and optimization of rare earth element chromatography. Doctoral dissertation, faculty of engineering. Lund University, Sweden
Eduafo PM (2013) Experimental investigation of recycling rare earth elements from waste fluorescent lamp phosphors. Colorado School of Mines
Cao X, Yin M, Wang X (2001) Elimination of the spectral interference from polyatomic ions with rare earth elements in inductively coupled plasma mass spectrometry by combining algebraic correction with chromatographic separation. Spectrochim Acta Part B At Spectrosc 56:431
Cassidy RM (1988) Determination of rare-earth elements in rocks by liquid Chromatography. Chem Geol 67:185
Potts PJ (1992) A handbook of silicate rock analysis. Blackie, Glasgow, p 610
Kumar M (2013) Recent trends in chromatographic procedures for separation and determination of rare earth elements, a review. Analyst 119:1994
Helfferich F (1962) Ion exchange. Mc Grow-Hill, New York
Abd El-Naby HH (2008) Genesis of secondary uranium minerals associated withjasperoid veins. El-Erediya area Eastern Desert, Egy J Min Depos 43:933–944
Abdallah SM (2004) Geological and mineralogical studies on some surface and subsurface sections from El-Missikat and El-Erediya uranium occurrences, Central Eastern Desert, Egypt. PhD Thesis. Ain-Shams University, Egypt
Osmond JK, Dabous AA, Dawood YH (1999) Uranium series age and origin of two secondary uranium deposits, Central Eastern Desert. Egypt Econ Geol 94:273
https://www.sigmaaldrich.com/catalog/product/sial/44443?lang=en®ion=EG. (2018). Accessed 18 Jan 2018
Marczenko Z, Balcerzak M (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier Science B.V, Amsterdam
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Langmuir (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. Am Chem Soc 40:1361–1368
Freundlich H (2009) Adsorption in solution. Phys Chem Soc 40:1361–1368
Othman H, Shabaan M, Demerdash M, Saleh M (2009) Experimental and theoretical investigation of sorption kinetics of beryllium on Amberlite-IR-120 sorbent. J Nucl Mater 392:427–433
Gonzalez-Luque S, Streat M (1983) Uranium sorption from phosphoric acid solutions using selective ion exchange resins: part I. Isotherms Extr Desorption Hydromet 11(2):207–225
Cortina JL, Miralles N (1997) kinetic studies on heavy metal ions removal by impregnated resins containing di-(2,4,4-trymethylpentyl) phosphinic acid. Solv Ext Ion Exch 15(6):1067–1083
Mohammed AA, Mahmood HS (2013) Removal of Cu+2, Pb+2 and Ni+2 ions from simulated waste water by ion exchange method on Zeolite and Purolite C105 resin. J Eng 19(10):1327
Rengaraj S, Yeon JW, Kim Y et al (2007) Adsorption characteristics of Cu (II) onto ion exchange resins 252H and 1500H: kinetics, isotherms and error analysis. J Hazard Mat 143(1–2):469
Kumar V, Jha MK, Kumari A et al (2014) Recovery of rare earth metals (REMs) from primary and secondary resources: a review. EPD Congress-2014, 16/02/2014 To 20/02/2014, (TMS). Diego Convention Center, San Diego
Pinto DVBS, Martins AH (2001) Electrochemical elution of a cation-exchange polymeric resin for yttrium and rare earth recovery using a statistical approach. Hydrometallurgy 60:99–104
Alstad J, Brunfelt AO (1967) Adsorption of the rare-earth elements on an anionexchange resin from nitric acid-acetone mixtures. Anal Chim Acta 38:185–192
Schijf J, Byrne RH (1999) Determination of stability constants for the mono- and difluoro-complexes of Y and the REE, using a cation-exchange resin and ICP-MS. Polyhedron 18:2839–2844
Dave SR, Kaur H, Menon SK (2010) Selective solid-phase extraction of rare earth elements by the chemically modified Amberlite XAD-4 resin with azacrown ether. React Funct Polym 70(9):692–698
Xiong C, Liu X, Yao C (2008) Effect of pH on sorption for RE(III) and sorption behaviors of Sm(III) by D152 resin. J Rare Earths 26(6):851–856
Kaur H, Agrawal YK (2005) Functionalization of XAD-4 resin for the separation of lanthanides using chelation ion exchange liquid chromatography. React Funct Polym 65:277–283
Suzuki T, Itoh K, Ikeda A et al (2006) Separation of rare earth elements by tertiary pyridine type resin. J Alloys Compd 408:1013–1016
Liu J, Yang X, Cheng X et al (2013) Synthesis and application of ion-imprinted polymer particles for solid-phase extraction and determination of trace scandium by ICP-MS in different matrices. Anal Methods 5:1811–1817
Zereen F, Yilmaz V, Arslan Z (2013) Solid phase extraction of rare earth elements in seawater and estuarine water with 4-(2-thiazolylazo) resorcinol immobilized Chromosorb 106 for determination by inductively coupled plasma mass spectrometry. Microchem J 110:178–184
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khawassek, Y.M., Eliwa, A.A., Haggag, E.S.A. et al. Adsorption of rare earth elements by strong acid cation exchange resin thermodynamics, characteristics and kinetics. SN Appl. Sci. 1, 51 (2019). https://doi.org/10.1007/s42452-018-0051-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0051-6