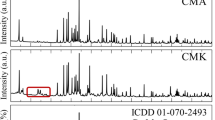
Abstract
Carbon monoxide (CO) is a poisonous atmospheric pollutant. It highly affects human beings, plants, animals and environment. Automobile exhaust is the largest source of CO emission in the environment. To control this automobile exhaust pollution, the catalytic converters are used. Many types of catalysts have been investigated for CO oxidation purposes, i.e., noble metal, base metal, rare earth, perovskite, spinel and mixed transient metal oxides. These catalysts are widely used in a catalytic converter. Among the various metal oxide catalysts, hopcalite (CuMnOx) is one of the most efficient catalysts for low-temperature CO oxidation. Hopcalite catalysts have been reported to be good from economical, thermal, activity, selectivity and availability points of view. The activity of hopcalite catalysts is strongly dependent on the surface area, crystallite size and binding energy of the catalysts. This study will provide a scientific basis for designing future application of hopcalite catalysts for low-temperature CO oxidation. This manuscript provides a summary of published information regarding pure and substituted hopcalite catalyst, synthesis methods; properties and application for CO emissions control. A number of papers associated with CO oxidation over the hopcalite catalysts have been available, but no review papers appear in the literature that is dedicated to CO oxidation. Therefore, in an attempt to fill this gap, the present review updates and evaluates the progress and future scope of hopcalite catalyst for purification of exhaust gases.

Source: missoula carbon monoxide emission inventory












Similar content being viewed by others
References
Aguila J, Chan N, Courtis J (1991) Proposed regulations for California phase 2 reformulated gasoline technical support document. California Air Resources Board, Sacramento
Air quality criteria for carbon monoxide (1991) Office of research and development. US Environmental Protection Agency, Washington (publication no. EPA-600/B-90/045F)
Alfuso S, Aurlemma M, Police G, Prati MV (1993) The effect of methyl-ester of rapeseed oil on combustion and emissions of di-diesel engines. SAE Paper 932801. SAE International, Warrendale
Amin CM, Rathod PP, Goswami JJ (2012) Copper based catalytic converter. Int J Eng Res Technol 1:1–6
Arana J, Piscina PR, Llorca J, Sales J, Homs N (1998) Bimetallic silica-supported catalysts based on Ni-Sn, Pd-Sn, and Pt-Sn as materials in the CO oxidation reaction. Chem Mater 10:1333–1342
Argyle MD, Bartholomew CH (2015) Heterogeneous catalyst deactivation and regeneration: a review. Catalysts 5:145–269
Automobile and carbon monoxide (2019) US environmental protection agency office mobile sources. EPA400-F-92-005, Fact sheet OMS-5, August 1994
Badr O, Probert SD (1994) Carbon monoxide concentration in the earth’s atmosphere. Appl Energy 49:99–143
Baltacioglu FS, Gulyuz B, Aksoylu AE, Onsan ZI (2007) Low temperature CO oxidation kinetics over activated carbon supported Pt-SnOx catalysts. Turk J Chem 31:455–464
Barbier J (1985) Effect of poisons on the activity and selectivity of metallic catalysts. In deactivation and poisoning of catalysts. Marcel Dekker, New York, pp 109–150
Bartholomew CH (2001) Mechanisms of catalyst deactivation. Appl Catal A 212:17–60
Behar S, Gonzalez P, Agulhon P, Quignard F, Swierczynski D (2012) New synthesis of nano sized Cu–Mn spinels as efficient oxidation catalysts. Catal Today 189:35–41
Benjamin BFF, Alphonse P (2016) Co-Mn-oxide spinel catalysts for CO and propane oxidation at mild temperature. Appl Catal B 180:715–724
Biemelt T, Wegner K, Trichert J, Lohe MR, Martin J, Grothe J, Kaskel S (2015) Hopcalite nanoparticle catalysts with high water vapour stability for catalytic oxidation of carbon monoxide. Appl Catal B 21:1–26
Cai L, Guo Y, Lu A, Branton P, Li W (2012) The choice of precipitant and precursor in the co-precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J Mol Catal A: Chem 360:35–41
Chand S, Sharma AK, Garg A, Mishra IM (2000) Supported perovskite catalysts for CO oxidation. J Sci Ind Res 59:944–948
Chauhan S (2010) Noble metal catalysts for monolithic converters. Journal of chemical and pharmaceutical research 4:602–611
Chen LWA, Doddridge BG, Dickerson RR, Chow JC, Mueller PK, Quinn J, Butler WA (2001) Seasonal variations in elemental carbon aerosol, carbon monoxide and sulfur dioxide; implications for sources. Geophys Res Lett 28:1711–1714
Chen H, Tong X, Li Y (2009) Mesoporous Cu–Mn Hopcalite catalysts and its performance in low temperature ethylene combustion in a carbon dioxide stream. Appl Catal A 370:59–65
Chhatwal GR, Mehra MC, Sataka M, Katyal T, Katyal M, Nagahiro T (1975) In environmental air pollution and its control. Anmol Publications, New Delhi
Choi K, Lee D, Kim H, Yoon Y, Park C, Kim YH (2016) Reaction characteristics of precious-metal-free ternary Mn−Cu−M (M = Ce Co, Cr and Fe) oxide catalysts for low-temperature CO oxidation. Ind Eng Chem Res 55:4443–4450
Cholakov GS (1999) Catalytic converters and other emissions control devices. Pollut Control Technol 3:1–8
Cholakov GS (2010) Control of exhaust emissions from internal combustion engine vehicles. Pollut Control Technol 3:1–8
Clarke TJ, Davies TE, Kondrat SA, Taylor SH (2015) Mechanochemical synthesis of copper manganese oxide for the ambient temperature oxidation of carbon monoxide. Appl Catal B 165:222–231
Cole KJ, Carley AF, Crudace MJ, Clarke M, Taylor SH, Hutchings GJ (2010) Copper manganese oxide catalysts modified by gold deposition: the influence on activity for ambient temperature carbon monoxide oxidation. Catal Lett 138:143–147
CONCAWE (1992) Environmental science for the European refining industry. The chemical composition of diesel particulate emissions. https://www.concawe.eu/
Cong H, Yu S (2009) Shape control of cobalt carbonate particles by a hydrothermal process in a mixed solvent: an efficient precursor to nanoporous cobalt oxide architectures and their sensing property. Cryst Growth Des 9:210–217
Dey S, Dhal GC, Prasad R, Mohan D (2016) Effect of nitrate metal (Ce, Cu, Mn and Co) precursors for the total oxidation of carbon monoxide. Res Eff Technol 3:293–302
Dey S, Dhal GC, Mohan D, Prasad R (2017a) Characterization and activity of CuMnOx/γ-Al2O3 catalyst for oxidation of carbon monoxide. Mater Discov 8:26–34
Dey S, Dhal GC, Mohan D, Prasad R (2017b) Copper based mixed oxide catalysts (CuMnCe, CuMnCo and CuCeZr) for the oxidation of CO at low temperature. Mater Discov 10:1–14
Dey S, Dhal GC, Mohan D, Prasad R (2017c) Effect of preparation conditions on the catalytic activity of CuMnOx catalysts for CO Oxidation. Bull Chem React Eng Catal 12(3):437–451
Dey S, Dhal GC, Prasad R, Mohan D (2017d) Effects of doping on the performance of CuMnOx catalyst for CO oxidation. Bull Chem React Eng Catal 12(3):1–14
Dey S, Dhal GC, Mohan D, Prasad R (2017e) Kinetics of catalytic oxidation of carbon monoxide over CuMnAgOx catalyst. Mater Discov 8:18–25
Dey S, Dhal GC, Mohan D, Prasad R (2017f) Study of Hopcalite (CuMnOx) catalysts prepared through a novel route for the oxidation of carbon monoxide at low temperature. Bull Chem React Eng Catal 12(3):393–407
Dey S, Dhal GC, Mohan D, Prasad R, Gupta RN (2018a) Cobalt doped CuMnOx catalysts for the preferential oxidation of carbon monoxide. Appl Surf Sci 441:303–316
Dey S, Dhal GC, Mohan D, Prasad R (2018b) Low-temperature complete oxidation of CO over various manganese oxide catalyst. Atmos Pollut Res 9:755–763
Dey S, Dhal GC, Mohan D, Prasad R (2019a) Synthesis of silver promoted CuMnOx catalyst for ambient temperature oxidation of carbon monoxide. J Sci 4:47–56
Dey S, Dhal GC, Mohan D, Prasad R (2019b) Ambient temperature complete oxidation of carbon monoxide using hopcalite catalysts for fire escape mask applications. Adv Compos Hybrid Mater 1:1–23. https://doi.org/10.1007/s42114-019-00108-5
Dissanayake DP (2006) Applications of metal oxides for volatile organic compound combustion, chap 17. In: Fierro JLG (ed) Metal Oxides Chemistry and Applications. Taylor and Francis group, p 26
Dvorak R, Chlapek P, Jecha D, Puchyr R, Stehlik P (2010) New approach to common removal of dioxins and NOx as a contribution to environmental protection. J Clean Prod 18:881–888
Einaga H, Nasu Y, Oda M, Saito H (2016) Catalytic performances of perovskite oxides for CO oxidation under microwave irradiation. Chem Eng J 283:97–104
EPA (2015) Air act, US summary of the clean air act. http://www.epa.gov. Retrieved 2015-12-22
Everaert K, Baeyens J (2004) The catalytic oxidation of hydrocarbon volatile organic compounds. J Hazard Mater B 109:113–139
Faiz A, Weaver SC, Walsh MP (1996) Air pollution from motor vehicles (Standards and Technologies for Controlling Emissions). The world bank, Washington
Fang D, Xie J, Mei D, Zhang Y, He F, Liu X, Li Y (2014) Effect of CuMn2O4 spinel in Cu–Mn oxide catalysts on selective catalytic reduction of NOx with NH3 at low temperature. RSC Adv 49:25540–25551
Favez J, Weilenmann M, Stilli J (2009) Cold start extra emissions as a function of engine stop time: evolution over the last 10 years. Atmos Environ 43:996–1007
Feaviour MR, Schofield EM (2007) Scientific bases for the preparation of heterogeneous catalysts. Platin Met Rev 51:42–44
Figuerido JL (ed) (1982) Progress in catalyst deactivation. NATO advanced study institute series E. Marunus Nijhoff, Boston
Fortunato G, Oswald HR, Reller A (2000) Spinel-type oxide catalysts for low temperature CO oxidation generated by use of an ultrasonic aerosol pyrolysis process. J Mater Chem 11:905–911
Fuzhen Z, Miao G, Guangying Z, Jinlin L (2015) Effect of the loading content of CuO on the activity and structure of CuO/Ce-Mn-O catalysts for CO oxidation. J Rare Earths 330:604–610
Gao Z, Kim M, Choi J, Daw CS, Parks JE, Smith DE (2012) Cold-start emissions control in hybrid vehicles equipped with a passive adsorber for hydrocarbons and nitrogen oxides. J Automob Eng 226(10):1396–1407
Gardner SD, Hoflund GB (1991) Catalytic behavior of noble metal/reducible oxide comparison of catalyst performance materials for low-temperature CO oxidation, comparison of catalyst performance. Am Chem Soc 91:2135–2140
Ghaffari A, Shamekhi AH, Saki A, Kamrani E (2008) Adaptive fuzzy control for Air-Fuel ratio of automobile spark ignition engine. World Acad Sci Eng Technol 48:284–292
Goldsmith JR, Aronow WS (1975) Carbon monoxide and coronary heart disease: a review. J Environ Res 10:236–248
Guo X, Li J, Zhou R (2016) Catalytic performance of manganese doped CuO–CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. J Fuel 20:56–64
Haruta M, Yamada N, Kobayashi T, Lijiama S (1989) Gold catalysts prepared by co-precipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J Catal 115:301–309
Hasegawa Y, Maki R, Sano M, Miyake T (2009) Preferential oxidation of CO on copper-containing manganese oxides. Appl Catal A 371:67–72
Hasunuma H, Ishimaru Y, Yoda Y, Shima M (2014) Decline of ambient air pollution levels due to measures to control automobile emissions and effects on the prevalence of respiratory and allergic disorders among children in Japan. Environ Res 131:111–118
Hoshyar N, Irankhak A, Jafari M (2015) Copper catalysts supported on CeMnO2 for CO oxidation in hydrogen rich gas streams. Iran J Chem Eng 12:3–14
Houshmand D, Roozbehani B, Badakhshan A (2013) Thermal and catalytic degradation of polystyrene with a novel catalyst. Int J Sci Emerg Technol 5:234–238
Hu Y, Dong L, Wang J, Ding W, Chen Y (2000) Activities of supported copper oxide catalysts in the NO + CO reaction at low temperatures. J Mol Catal A: Chem 162:307–316
Huang T, Tsai D (2003) CO oxidation behavior of copper and copper oxides. Catal Lett 87:173–178
Hutchings GJ, Mirzaei AA, Joyner RW, Siddiqui MRH, Taylor SH (1996) Ambient temperature CO oxidation using copper manganese oxide catalysts prepared by co-precipitation: effect of ageing on catalyst performance. Catal Lett 42:21–24
Hutchings GJ, Mirzaei AA, Joynerb RW, Siddiqui MRH, Taylor SH (1998) Effect of preparation conditions on the catalytic performance of copper manganese oxide catalysts for CO oxidation. Appl Catal A 166:143–152
India Bharat Stage VI emission standards (2016) Policy update. International Council of Clean Transportation, Washington
Irawan RB, Purwanto P, Hadiyanto H (2015) Optimum design of manganese-coated copper catalytic converter to reduce carbon monoxide emissions on gasoline motors. Int Conf Trop Coast Reg Eco-Dev 23:86–92
Ismaila SO, Bolaji BO, Adetunji OR, Adekunle NO, Yusuf TA, Sanusi HO (2013) On vehicular emissions of petrol and diesel engines. Int J Eng 1584:178–180
Ivanov KI, Kolentsova EN, Dimitrov DY, Avdeev GY, Tabakova TT (2015) Alumina supported copper-manganese catalysts for combustion of exhaust gases: catalysts characterization. Int Sch Sci Res Innov 9(6):719–724
Jackson NB, Datye AK, Mansker L, O’Brien RJ, Davis BH (1997) Deactivation and attrition of iron catalysts in synthesis gas in catalyst deactivation. Stud Surf Sci Catal 111:501–509
Jansson J (2000) Low-temperature CO oxidation over Co3O4/Al2O3. J Catal 194:55–60
Jin M, Park J, Shon JK, Kim JH, Li Z, Park Y, Kim JM (2012) Low temperature CO oxidation over Pd catalysts supported on highly ordered mesoporous metal oxides. Catal Today 185:183–190
Jones C, Taylor SH, Burrows A, Crudace MJ, Kielyb CJ, Hutchings GJ (2008) Cobalt promoted copper manganese oxide catalysts for ambient temperature carbon monoxide oxidation. Chem Commun 1707:1–7
Jones C, Cole KJ, Taylor SH, Crudace MJ, Hutchings GJ (2009) Copper manganese oxide catalysts for ambient temperature carbon monoxide oxidation: effect of calcination on activity. J Mol Catal A: Chem 305:121–124
Kam EKT, Hughes R (2001) The effect of catalyst fouling on the performance of adiabatic packed-bed reactors—a theoretical study. Chem Eng J 18:93–102
Katara P (2015) Review paper on catalytic converter for automobile exhaust emission. Int J Sci Res (IJSR) 5:30–33
Kireev AS, Mukhin VM, Kireev SG, Klushin VN, Tkachenko SN (2009) The preparation and properties of modified hopcalite catalyst. Russ J Appl Chem 82:169–171
Kondrat SA, Davies TE, Zu Z, Boldrin P, Bartley JK, Carley AF, Taylor SH, Rosseinsky MJ, Hutchings GJ (2011) The effect of heat treatment on phase formation of copper manganese oxide: influence on catalytic activity for ambient temperature carbon monoxide oxidation. J Catal 281:279–289
Kramer M, Schmidt T, Stowe K, Maier WF (2006) Structural and catalytic aspects of sol–gel derived copper manganese oxides as low-temperature CO oxidation catalyst. Appl Catal A 302:257–263
Larsson P, Andersson A (2000) Oxides of copper, ceria promoted copper, manganese and copper manganese on Al2O3 for the combustion of CO, ethyl acetate and ethanol. Appl Catal B 24:175–192
Lee J, Kim H, Lee H, Jang S, Chang JH (2016) Highly efficient elimination of carbon monoxide with binary copper-manganese oxide contained ordered nanoporous silicas. Nanoscale Res Lett 11:2–6
Li M, Wang D, Shi X, Zhang Z, Dong T (2007) Kinetics of catalytic oxidation of CO over copper-manganese oxide catalyst. Sep Purif Technol 57:147–151
Li X, Dai H, Deng J, Liu Y, Xie S, Zhao Z, Wang Y, Guo G, Arandiyan H (2013) Au/3DOM LaCoO3: high-performance catalysts for the oxidation of carbon monoxide and toluene. Chem Eng J 228:965–975
Liu Q, Liu C, Nie X, Bai L, Wen S (2012) Facile synthesis of mesoporous CO3O4 via a soft reactive grinding route and their application in the CO oxidation. Mater Lett 72:101–103
Liu Y, Guo Y, Peng H, Xu X, Wu Y, Peng C, Zhang N, Wang X (2016) Modifying hopcalite catalyst by SnO2 addition: an effective way to improve its moisture tolerance and activity for low temperature CO oxidation. Appl Catal A 525:204–214
Makwana NR, Amin CM, Dabhi SK (2013) Development and performance analysis of nickel based cobalt catalyst. Int J Adv Eng Technol 4(2):10–13
Marino F, Descorme C, Duprez D (2004) Noble metal catalysts for the preferential oxidation of carbon monoxide in the presence of hydrogen (PROX). Appl Catal B 54:59–66
Marino F, Descorme C, Duprez D (2005) Supported base metal catalysts for the preferential oxidation of carbon monoxide in the presence of excess hydrogen (PROX). Appl Catal B 58:175–183
Marinoiu A, Raceanu M, Cobzaru C, Teodorescu C, Marinescu D, Soare A, Varlam M (2014) Low temperature CO retention using hopcalite catalyst for fuel cell applications. React Kinet Mech Catal 112:37–50
Martino, Wakim, Welstand (1994) Comparison of HC and CO emissions estimates using FTP, IM240, and remote sensing. Paper presented at the 4th CRC On-Road Vehicle Emissions Workshop, March 16–18 1994, San Diego, California
Mirzaei AA, Shaterian RH, Habibi M, Hutchings GJ, Taylor SH (2003) Characterization of copper-manganese oxide catalysts: effect of precipitate ageing upon the structure and morphology of precursors and catalysts’. Appl Catal A 253:499–508
Mirzaei AA, Shaterian HR, Joyner RW, Stockenhuber M, Taylor SH, Hutchings GJ (2013) Ambient temperature carbon monoxide oxidation using copper manganese oxide catalysts: effect of residual Na+ acting as catalyst poison. Catal Commun 4:17–20
Mishra A, Prasad R (2014) Preparation and application of perovskite catalysts for diesel soot emissions control: an overview. Catal Rev: Sci Eng 56:57–81
Mokhtar M, Basahel SN, Al-Angary YO (2010) Nanosized spinel oxide catalysts for CO-oxidation prepared via CoMnMgAl quaternary hydrotalcite route. J Alloy Compd 493:376–384
Morales MR, Barbero BP, Cadus LE (2006) Total oxidation of ethanol and propane over Mn–Cu mixed oxide catalysts. Appl Catal B 67:229–236
Nisar J, Ali M, Awan IA (2011) Catalytic thermal decomposition of polyethylene by pyrolysis gas chromatography. J Chil Chem Soc 56:653–654
Njagi EC, Chen C, Genuino H, Galindo H, Huang H, Suib SL (2010) Total oxidation of CO at ambient temperature using copper manganese oxide catalysts prepared by a redox method. Appl Catal A 99:103–110
Njagi EC, Genuino HC, Kingondu CK, Chen C, Horvath D, Suib SL (2011) Preferential oxidation of CO in H2 rich feeds over mesoporous copper manganese oxide synthesized by a redox method. Int J Hydrogen Energy 36:6768–6779
Paldey S, Gedevanishvili S, Zhang W, Rasouli F (2005) Evaluation of a spinel based pigment systems as a CO oxidation catalyst. Appl Catal B 56:241–250
Papavasiliou J, Avgouropoulos G, Ioannides T (2005) Steam reforming of methanol over copper–manganese spinel oxide catalysts. Catal Commun 6:497–501
Patel F, Patel S (2013) La1-XSrXCoO3 (x = 0, 0.2) perovskites type catalyst for carbon monoxide emission control from auto-exhaust. Procedia Eng 51:324–329
Peng CT, Lia HK, Liaw BJ, Chen YZ (2011) Removal of CO in excess hydrogen over CuO/Ce1−XMnxO2 catalysts. Chem Eng J 172:452–458
Perego C, Villa P (1977) Catalysts preparation methods. Catal Today 34:281–305
Prockop LD, Chichkova RI (2007) Carbon monoxide intoxication: an updated review. J Neurol Sci 262:122–130
Puckhaber LS, Cheung H, Cocke DL, Clearfield A (1989) Reactivity of copper manganese oxides. Solid State Ionics 32:206–213
Pulkrabek WW (2004) Engineering fundamentals of the internal combustion engine. Pearson Prentice Hall, New Jersey
Qian K, Qian Z, Hua Q, Jiang Z, Huang W (2013) Structure activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl Surf Sci 273:357–363
Rani R, Prasad R (2014) Studies of carbon monoxide oxidation at ambient conditions. Recent Res Sci Technol 6:89–92
Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR (2000) Carbon monoxide poisoning—a public health perspective. J Toxicol 145:1–14
Richards HM, MacDougal DT (1904) The influence of carbon monoxide and other gases upon plants. Bull Torrey Bot Club 31:57–66
Rossetti I, Buchneva O, Biffi C, Rizza R (2009) Effect of sulphur poisoning on perovskite catalysts prepared by flame-pyrolysis. Appl Catal B 89(3–4):383–390
Rostrup-Nielsen JR (1991) Promotion by poisoning In catalyst deactivation. Stud Surf Sci Catal 68:85–101
Royer S, Duprez D (2011) Catalytic oxidation of carbon monoxide over transition metal oxides. Chemcatchem 3:24–65
Rudolf W (1994) Concentration of air pollutants inside cars driving on highways and in downtown areas. Sci Total Environ 146(147):433–444
Santra AK, Goodman DW (2002) Catalytic oxidation of CO by platinum group metals: from ultrahigh vacuum to elevated pressures. Electrochim Acta 47:3595–3609
Schubert MM, Kahlich MJ, Feldmeyer G, Hackenberg MS, Gasteiger HA, Behm RJ (2001) Bimetallic PtSn catalyst for selective CO oxidation in gases H2-rich at low temperatures. Phys Chem Chem Phys 3:1123–1131
Schwab GM, Kanungo SB (1977) Efficient stable catalyst for low-temperature carbon monoxide oxidation. J Catal 107:109–120
Severino F, Brito JL, Laine J, Fierro JLG, Agudo AL (1998) Nature of copper active sites in the carbon monoxide oxidation on CuAl2O4 and CuCr2O4 spinel type catalysts. J Catal 177:82–95
Sharaf J (2013) Exhaust emissions and its control technology for an internal combustion engine. Int J Eng Res Appl 3:947–960
Shi L, Hu Z, Deng G, Li W (2015) Carbon monoxide oxidation on copper manganese oxides prepared by selective etching with ammonia. Chin J Catal 36:1920–1927
Singh P, Prasad R (2014) Catalytic abatement of cold-start vehicular CO emissions. Catal Ind 6:122–127
Solsona B, Hutchings GJ, Garcia T, Taylor SH (2004) Improvement of the catalytic performance of CuMnOx catalysts for CO oxidation by the addition of Au. New J Chem 28:708–711
Spivey JJ (1987) Complete catalytic oxidation of volatile organics. Am Chem Soc 26:2165–2180
Tanaka Y, Utaka T, Kikuchi R, Takeguchi T, Sasaki K, Eguchi K (2003) Water gas shift reaction for the reformed fuels over Cu/MnO catalysts prepared via spinel-type oxide. J Catal 215:271–278
Tang ZR, Kondrat SA, Dickinson C, Bartley JK, Carley AF, Taylor SH, Davies TE, Allix M, Rosseinsky MJ, Claridge JB, Xu Z, Romani S, Crudace MJ, Hutching GJ (2011) Synthesis of high surface area CuMn2O4 by supercritical anti-solvent precipitation for the oxidation of CO at ambient temperature. Catal Sci Technol 1:740–746
Tavares MT, Bernardo CA, Alstrup I, Rostrup-Nielsen JR (1986) Reactivity of carbon deposited on nickel-copper alloy catalysts from the decomposition of methane. J Catal 100:545–548
Taylor SH, Hutchings GJ, Mirzaei AA (1999) Copper zinc oxide catalysts for ambient temperature carbon monoxide oxidation. Chem Commun 15:1373–1374
The California low-emission vehicle regulations (2010) With amendments effective. pp 1–134. https://ww3.arb.ca.gov/msprog/levprog/cleandoc/cleancomplete_lev-ghg_regs_12-10.pdf
Thormahlen P, Skoglundh M, Fridell E, Andersson B (1999) Low-temperature CO oxidation over platinum and cobalt oxides catalysts. J Catal 188:300–310
Votsmeier M, Kreuzer T, Lepperhoff G (2005) Automobile exhaust control. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–15
Wojciechowska M, Przystajko W, Zielinski M (2007) CO oxidation catalysts based on copper and manganese or cobalt oxides supported on MgF2 and Al2O3. Catal Today 119:338–341
Xuan W, Xu S, Yuan X, Shen W (2008) Carbon monoxide—a novel and pivotal signal molecule in plants. J Plant Signal Behav 3:381–382
Yao YY (1983) The oxidation of CO and hydrocarbons over noble metal catalysts. J Catal 87:152–162
Yap YH, Lim MSW, Lee ZY, Lai KC, Jamaal MA, Wong FH, Ng HK, Lim SS, Tiong TJ (2018) Effects of sonication on co-precipitation synthesis and activity of copper manganese oxide catalyst to remove methane and sulphur dioxide gases. Ultraso Sonochem 40:57–67
Yasar A, Haider R, Tabinda AB, Kausar F, Khan M (2013) A comparison of engine emissions from heavy, medium and light vehicles for CNG, diesel and gasoline fuels. Pol J Environ Stud 22:1277–1281
Zaki MI, Hasan MA, Pasupulety L (2009) Influence of CuOx additives on CO oxidation activity and related surface and bulk behaviors of Mn2O3, Cr2O3 and WO3 catalysts. Appl Catal A 198:247–259
Zhang X, Ma K, Zhang L, Yong G, Dai Y, Liu S (2010) Effect of precipitation method and Ce doping on the catalytic activity of copper manganese oxide catalysts for CO oxidation. Chin J Chem Phys 24:97–102
Zhao K, Tang H, Qiao B, Li L, Wang H (2014) High activity of Au/γ-Fe2O3 for CO Oxidation: effect of support crystal phase in catalyst design. ACS Catal 5:3528–3539
Zheng X, Wang X, Yu L, Wang S, Wu S (2006) Base metal catalysts in carbon monoxide oxidation. Prog Chem 18:159–167
Zhengqian L, Jun MA, Lei Z (2007) Effect of preparation parameters on catalytic properties of Pt/graphite. Front Environ Sci Eng 4:482–487
Zhou Y, Wang Z, Liu C (2014) Perspective on CO oxidation over Pd-based catalysts. Catal Sci Technol 5:69–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dey, S., Dhal, G.C. A Review of Synthesis, Structure and Applications in Hopcalite Catalysts for Carbon Monoxide Oxidation. Aerosol Sci Eng 3, 97–131 (2019). https://doi.org/10.1007/s41810-019-00046-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41810-019-00046-1