Abstract
Antibody-dependent enhancement (ADE) is an alternative route of viral entry in the susceptible host cell. In this process, antiviral antibodies enhance the entry access of virus in the cells via interaction with the complement or Fc receptors leading to the worsening of infection. SARS-CoV-2 variants pose a general concern for the efficacy of neutralizing antibodies that may fail to neutralize infection, raising the possibility of a more severe form of COVID-19. Data from various studies on respiratory viruses raise the speculation that antibodies elicited against SARS-CoV-2 and during COVID-19 recovery could potentially exacerbate the infection through ADE at sub-neutralizing concentrations; this may contribute to disease pathogenesis. It is, therefore, of utmost importance to study the effectiveness of the anti-SARS-CoV-2 antibodies in COVID-19-infected subjects. Theoretically, ADE remains a general concern for the efficacy of antibodies elicited during infection, most notably in convalescent plasma therapy and in response to vaccines where it could be counterproductive.
Similar content being viewed by others
1 Introduction
With the emergence of the SARS-CoV-2 pandemic in December 2019 in the Wuhan province of China as its epicenter, it brought a wave of morbidity and mortality worldwide, spreading rapidly to more than 190 nations infecting over 180 million people1,2,3,4,5. SARS-CoV-2 is a betacoronavirus that is zoonotic in nature, causes pulmonary infection, and infects the upper or lower respiratory tract6. This virus shares a sequence similarity of 79% with SARS-CoV and about 50% with MERS-CoV at the whole genome level7,8,9. A spectrum of clinical signs and symptoms have been observed for COVID-19 patients ranging from mild symptoms like fever, cough, sore throat, loss of taste and smell to severe form of illness10,11,12. People with underlying comorbidities and elderly populations seem to be at heightened risk and more susceptible to infection, including other complications13,14.
Coronaviruses gain entry into the cell upon binding to the cellular receptor for angiotensin-converting enzyme 2 in the epithelial cells (ACE2) through the viral spike(S) protein8,9; this is followed by S protein priming by host cell surface protease, the serine protease TMPRSS28,15. The S protein of SARS-CoV-2 consists of the S1 subunit, which has a receptor-binding domain (RBD), and the S2 subunit that mediates membrane fusion for viral entry1. Currently, the vaccines available for SARS-CoV-2 target the RBD region of S protein for preventing its attachment and thus infection via blocking RBD-ACE2 interaction16–17,18,19,20,21,22,.
When whole antibodies elicited in viral infection at a suboptimal concentration that is generally expected to be protective, provide an advantage to the virus by giving entry access to the cells that could lead to the enhancement of the infection called antibody-dependent enhancement (ADE), then the risk of aggravating the severity of infection increases. SARS-COV-2 infection leads to the production of neutralizing antibodies, but the extent to which these antibodies would be neutralizing and protective to the subsequent SARS-CoV-2 infection is still not clear. Some reports of SARS-COV-2 reinfection have been published, elucidating illness with the genetically distinct strain of SARS-CoV-2 with symptomatic reinfection24,25,26. There is a possibility of severe cases of infection during a secondary infection with SARS-COV-2, similar to DENV serotypes as reported previously, due to the risk of cross-reactive antibodies potentially capable of promoting ADE. This cross-reactivity of antibodies with different strains may give rise to the phenomenon of ADE and make the symptoms more severe; acute respiratory distress syndrome (ARDS) is majorly attributed to the severe cases of illness that have emerged to be the leading cause of death in COVID-19, similar to SARS and MERS6.
Even though the link between ADE of infection and disease severity is yet to be established, in the past, the severity of infection and cross-reactivity of antibodies to other viral serotypes have been linked and established in vitro for Zika, Dengue, and Influenza A viral infection28,29,30. These previous and current studies point towards the requirement of proactive research in the immunopathology caused by COVID-19. Because a large and variable group of people are getting infected with severity ranging from mild to severe cases of illness, therefore, the possibility of ADE is worth considering.
2 ADE in Related Organism: Dengue
Dengue virus (DENV) is a single positive-stranded RNA virus of the family Flaviviridae and genus Flavivirus. ADE has been well documented in this disease. Dengue fever is caused by four antigenically distinct dengue virus serotypes (DENV 1–4). Live attenuated vaccine (LAV) for Dengue raised a serious concern due to their capacity to elicit an adverse immune response. The concern behind this limited approval is that this vaccine predisposed some of the dengue-naïve recipients to severe dengue fever due to their cross-reactivity with other DENV serotypes, thereby contributing to ADE. Therefore, the major bottleneck with whole DENV-based vaccine strategies may be overcome using an antibody response serotype. ADE may be a significant concern in the case of COVID-19, not just due to a suboptimal response to different variant of concern (VoC) but also due to an inadequate viral neutralization.
In this review, we aim to assess the hypothesis that non-neutralizing antibodies or antibodies that are neutralizing but possess a low affinity to critical regions of virus entry points may be associated with the severity of infection in COVID-19 that fails to neutralize the virus. These antibodies may be formed by infection or vaccination with a closely related serotype of SARS-CoV-2, previous exposure to other classes of coronaviruses. In this review, we discuss the phenomenon of ADE, its possible mechanism that may play a significant role in the pathogenesis of COVID-19 by relating the previous and some current findings with the COVID-19 pandemic and highlights the interplay of antibodies that may be neutralizing or non-neutralizing in nature with viral surface receptors that may lead to this condition.
3 Antibody-Dependent Enhancement Phenomenon
Virus entry into host cells is a primary obligate process in viral pathogenesis; this is usually mediated by hijacking the cellular mechanism. Neutralizing antibodies aid in inhibiting the attachment of the virus to the host cell receptors by targeting the viral surface proteins or glycoproteins and is considered to be a crucial mechanism to eliminate the virus31,32,33,34,35,36, and the attachment between viruses and target cells plays an essential role in most cases and may produce different outcomes. However, in some instances, paradoxically, binding of the antibodies at sub‐neutralizing concentrations to non-critical sites of the virion can result in non-neutralization of the virion that may lead to virion entry and invasion into certain cell types via antibody Fc region present on the carboxyl-terminal domain of antibody with Fc gamma receptor IIa (FcRIIa)-expressing phagocytic cells like the monocytes, macrophages, or through interaction with complement receptors contributing in the enhancement of infection, a process known as antibody-dependent enhancement37,38,39,40,41 during these instances, the binding of antibodies to these non-critical sites leaves the virus with retainment of its infectivity37. Virus possesses various kinds of different antigenic epitopes capable of inducing neutralizing antibodies, but some might induce non-neutralizing antibodies that may enhance the infection42. Despite the presence of multiple critical sites on the virus surface, immunoglobulins, after attaching to this area, may not neutralize the virus completely because the virus can utilize another site for interacting with the host cell 37. Diluting concentrations of antibodies have been shown to increase lung pathology and infiltration of cells into the alveolar air space observed in vivo mouse model during the influenza virus life cycles 43. In an experimental study, it was observed that lower levels of IgG could promote the uptake of human parvovirus (B19V) in endothelial cells showing an enhancing effect of lower levels of antibodies at the level of virus internalization44. Seropositive people who may have successfully eliminated one viral serotype may well be at increased risk of infection with other viral serotypes. The neutralizing antibodies preformed for one of these serotypes might often not be neutralizing for different serotypes that may cross-neutralize the epitopes, and these deficient and incompetent neutralizing antibodies may instead allow ADE mechanism to kick in, leading to enhanced infection 45,46,47. Dengue viruses, one of the best studied, representing a classic example of flaviviruses exhibiting ADE cause infection through four distinct serotypes DENV1, DENV2, DENV3, and DENV4; antibodies raised for one Dengue serotype do not protect against other serotypes failing to block the virus entry into cells42,48,49; the humoral response produced against one Dengue serotype provides protective serotype‐specific antibodies; however, these antibodies do cross‐react with other Dengue viral serotypes but do not neutralize them that may promote the entry of the virus via antibody Fc regions failing in protecting against different viral serotypes (Fig. 1); as a result, a second Dengue viral infection could arise, being more lethal, leading to dengue hemorrhagic fever and shock syndrome42,50, this similar instance of ADE can happen in COVID-19 in which RBD region of S protein of SARS-CoV-2 may get mutated as found in some studies51,52. Enhancement of disease and more severity has been described in infants who received inactivated respiratory syncytial virus (RSV) vaccine as well as inactivated measles vaccine after they encountered a secondary viral infection53,54.
Antibody-dependent enhancement in DENV. a Primary infection of DENV induces neutralizing antibodies at sufficient concentration that potentially neutralizes and destroys DENV providing protective immunity. b In secondary infection, neutralizing antibodies that are elicited successfully neutralize the virus when the DENV serotype is similar to the primary DENV infection. c Antibody-dependent enhancement of infection in DENV infection occurs when non-neutralizing antibodies formed from primary infection bind with different DENV serotypes during secondary infection, these Ab from a primary infection are unable to neutralize the different DENV serotype that enhances the virus entry and replicate into cells leading to a heightened risk of dengue viral infection severity. Image credit smart.servier.com.
4 Mechanism of ADE
The virus–antibody complex binds with either Fc or complement receptors expressed on immune cells leading to the internalization of the virus–antibody complex that must follow the destruction of the virus. However, in some instances, the virus escapes the antigen–antibody complex and starts a replication cycle inside immune cells that possibly occurs when the virus is bound to low-affinity antibodies38. Although the exact mechanism of ADE remains to be understood, ADE has been reported to take place in two possible ways, first, internalization of virus–antibody immune complexes into phagocytic cells via interaction of the antibody Fc region with the cellular Fc receptors present on myeloid cells which then render immune system to trigger signal transduction, releasing inflammatory cytokines, superoxide burst, and antibody-dependent cell-mediated cytotoxicity (ADCC) leading to the heightened antibody-mediated uptake of the virus27,38,55 (Fig. 2). This type of Fc-dependent mechanism has been documented in West Nile virus, dengue virus, and human immunodeficiency virus 42. Fc receptors have been shown to play a pivotal in promoting antibody-dependent cell enhancement mechanisms38. Generally, cells that express Fc receptors lead to phagocytosis of antigen–antibody complexes as well as the direct killing of target cells by a process known as Antibody-dependent cellular cytotoxicity (ADCC)56. However, type 1 FcγR receptors are expressed by myeloid lineage cells such as monocytes, macrophages, dendritic cells, granulocytes including neutrophils and eosinophils, B and NK cells57, ADE is primarily observed in monocytes, macrophages, and dendritic cells 58,59,60,61. CD32(FcγRII) in monomeric form has a low affinity for the Fc region of IgG antibodies but possesses a high affinity for IgG immune complexes62. FcγRI (CD64) binds with monomeric IgG with high affinity63,64. The second possible mechanism of ADE is the complement-mediated enhancement of infection by complement protein C1q that is activated in the classical pathway or C3 that is activated in an alternative pathway followed by the binding of the antibody to the viral surface proteins forming the complex of virus-antibody-complement protein65 (Fig. 3). C1q binds to the Fc region of IgG1 and IgM that are complement-fixing antibodies attached to the viral proteins65 followed by the interaction between the corresponding receptor and complement protein that increases viral adhesion leading to the formation of the virus, antibody, and complement complex42,66. ADE mediated by complement protein CIq has been found with Ebola virus in non‐monocytic cells by endocytosis or enhancing virus attachment to the target cell; thus, this resulting complex consisting of virus-antibody-C1q binds to C1q cell surface receptors leading to either the binding of the virus to Ebola-specific receptors or endocytosis via C1q receptors67,68,69. The involvement of complement protein C1q has also been shown to mediate HIV-1 infection by binding to the Fc portion of antibodies that enhanced infection in vitro as immunocomplex as C1q binds with C1q receptors at the cell surface38,70. C3-mediated ADE is found in both the West Nile and HIV virus. Principally, IgG antibodies have been observed to mediate ADE; however, IgM as well as IgA along with Complement, have also been shown to be capable of ADE71,72; for instance, IgM-dependent enhancement of West Nile virus mediated by complement protein CR3 has been found in macrophage immune cells73.
Fc-dependent mechanism of ADE. a Non-neutralizing antibodies enhancing viral infection through Fc-dependent pathway as a result of failure to block binding of virus-specific receptors to host cell receptor. As the Fc region of non-neutralizing antibodies bind with viral spike protein epitope at sites other than the receptor binding with the FcγR on myeloid cells such as macrophage, phagocytosis occurs, resulting in an increased number of virus particles. b Entry of virus occurs through clathrin-coated vesicles through FcγRII receptors. Created with BioRender.com.
Complement-mediated pathway of ADE. a Binding of non-neutralizing antibodies to the viral spike protein forming an antigen–antibody complex formed by either IgG or IgM. b The formation of the Immune complex activates the classical pathway of the complement system. The Ag–Ab complex induces a conformational change in the Fc region of an antibody that exposes the binding site for complement proteins to bind to the Fc region. c Complement protein C1q/C3 attached to Ag–Ab complex binds with complement receptor on macrophage leading to the d internalization of Virus particles enhancing infection as a result of replication of the virus. Created with BioRender.com.
5 Neutralizing Antibodies and ADE
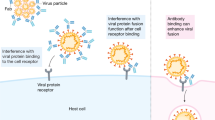
As the virus utilizes its envelope proteins to attach to the target cell surface receptors or coreceptors, neutralizing antibodies targeted against viral proteins generally hinder this step by targeting critical regions of the viral proteins preventing the binding of the virus (Fig. 4), this, in turn, reduces the infectivity of the virus leading to its neutralization37. The production of neutralizing antibodies is the desirable primary goal of vaccination, and therefore, antibodies that are secreted are expected to be neutralizing 74. Neutralizing antibodies are secreted as part of the humoral response of the active immune system mediated by Ab secreting plasma cells. Pathogens disarmed by these antibodies are generally phagocytosed by macrophages; neutralization of viral infectivity can take place in some ways; they may either interfere with the binding of the virion to the cellular receptors, fusion with the host membrane in case of enveloped viruses, membrane penetration (for nonenveloped viruses), may block uptake into the cells, prevent uncoating of the genomes in the endosomes or cause aggregation of virus particles 37. Antiviral antibodies lyse the enveloped viruses and serum complement disrupt membranes 36,74,75. The neutralizing effect of antibodies depends on certain factors, for instance, the stoichiometry of antibody(Ab titer), that must exceed a particular threshold 75; the antibody affinity for viral epitopes regulates the fraction of epitopes on the viral particle occupied by antibodies at any given concentration that is referred to as occupancy that predominantly determines the neutralization potential, as well as the accessibility of epitope, are both obligatory to exceed the threshold requirements as the antibodies possessing poor accessibility to specific epitopes require a higher concentration to exceed the occupancy threshold for neutralization36,76. A similar observation has been made in a study, where77 the diluted form of antisera against SARS-CoV spike protein has been found to promote ADE and enhanced apoptosis at the same time, while neutralizing antibodies against SARS-CoV neutralized infection55; so, it is the avidity that is more important and not merely the affinity. An increasing body of evidence shows that cross-reactive antibodies can have a crucial impact but greatly varied, these cross-reactive antibodies can perform neutralization if they bind viral epitopes with higher affinity78,79,80; nonetheless, the longevity of neutralizing antibodies is found to be higher than cross-neutralizing antibodies; a study found neutralizing antibody IgG to last longer than cross-reactive antibody that declined over time specific against a dengue virus serotype81. Cross-neutralizing antibodies may bind to specific regions of antigen but fail to neutralize it. Similar results have shown that neutralizing antibodies (nAbs) when targeting SARS-CoV, binds to viral RBD epitope of the spike protein, but not the receptor-binding motif that culminated in the failure of cross-neutralization of infection 82,83. Kathleen et al. found that S-RBD-specific antibodies exhibited more neutralizing potential than N-protein-specific antibodies 84, this in vitro study explains that not all antibodies elicited are neutralizing. In an in vivo study, Syrian hamsters were tested for the efficacy of antibodies isolated from convalescent donors, it was found that despite their efficient binding to S and/or RBD proteins of SARS-CoV-2, antibodies not competitive with ACE2 failed to inhibit the virus from entering host cells85. The early presence of IgG subtypes has been observed in some patients86, indicative of a possible memory to a cross-reactive antigen in a secondary immune response that might increase disease severity due to ADE86,87. Mutations in the concerned viral proteins may render the immune system to preferentially utilize the immunological memory from a previous infection during the encounter with a slightly different strain of the virus and may boost non-neutralizing antibodies, these non-neutralizing antibodies may reduce the efficacy of vaccines and for this reason, the vaccine for Influenza requires to be developed every year87,88,89. Also, there is no evidence that the immune system elicits neutralizing antibodies during immune response against a pathogen over non-neutralizing ones90. Still, the factors responsible for an effective and long-lasting antibody response remains unclear.
Secretion of neutralizing antibodies and its mechanism of blocking of viral infection. a B cell with B-cell receptors (BCR). b Antigen binds with a B-cell receptor specific to this antigen. c Clonal selection of an antigen-activated B cell leads to a clone of effector B cells and memory B cells, these clone cells are specific to the attached antigen; plasma cells secrete antibody reactive with the activating antigen. d Neutralizing antibodies binding to the critical sites on viral spike proteins e. blocking sites of attachment to host cell receptor ACE2 preventing fusion between. Created with BioRender.com.
6 Evidence of ADE in Coronaviruses
In the first report of ADE in 1964, enhancement of the infectivity of arboviruses such as Murray Valley encephalitis virus, West Nile virus, and Japanese encephalitis virus was observed during their neutralization in the presence of chicken antisera, all of which belong to the family Flaviviridae91, it was found afterwards that the IgG antibodies in the sera were responsible for this enhancement27; however, no biological explanation was being given for this process of enhancement. Since then, flaviviruses have been intensively studied to elucidate ADE’s mechanisms and clinical significance in viral pathogenesis. Among flaviviruses, Dengue was the first virus in which ADE was clearly established in 197727, and the relationship between the secondary infection associated with antibody response and severe illness was recognized in Dengue viruses; studies showed that low concentrations of IgG Abs were able to enhance infection 30,39,47. A probable role of ADE was speculated by a mathematical model that related the disease severity with enhancing effect of cross-reactive antibody to different DENV serotypes during secondary infection92. In vitro studies of ADE have been observed for Flaviviruses such as Dengue virus, yellow fever virus, and zika virus; Coronaviruses including alpha and beta coronaviruses, orthomyxoviruses such as influenza viruses, retroviruses such as HIV and feline infectious peritonitis virus (FIPV), other viruses such as Coxsackievirus B, respiratory syncytial virus, Ebola virus, alphaviruses and rabies virus 27,28,67,93,94,95,96. The in vitro and in vivo studies done to demonstrate ADE in different viruses is summarised in Table 1.
In vivo studies done in rhesus monkeys reflecting the relationship between antibody response and increased Dengue viremia have added further evidence to ADE phenomenon103,118. Complement-mediated ADE has been extensively studied in HIV and West Nile virus 73,119. In vitro studies have shown that sera from convalescent patients from Ebola virus disease contain antibodies capable of promoting ADE 120. In vivo studies done in rhesus monkeys reflecting the relationship between antibody response and increased viremia to different DENV serotypes has been added to further evidence to the phenomenon of ADE 94,103,118,121,122. Similarly, in an experimental finding, enhanced yellow fever immunogenicity upon yellow fever vaccination was observed in subjects with a specific range of cross-reactive antibody titers from a previous inactivated Japanese encephalitis vaccination 123; similar observations have been made when antibodies elicited after vaccination against Japanese encephalitis virus were found to enhance dengue virus infection 124; a study in COVID-19-affected patients reported that the higher antibody titers against SARS-CoV-2 were associated with more severe disease that raises the possibility of antibody-dependent disease enhancement effect 125. A possible case of COVID-19 reinfection has been observed in a patient with two different COVID-19 infection who was found to be infected with two genetically different SARS-CoV-2 variants 126. ADE of SARS pathogenesis has been shown to occur in different circulating immune cell types such as monocytes and macrophages; however, upon the induction of ADE, macrophages did not show any fruitful replication or modification of expression of pro-inflammatory cytokines or chemokines 127. In a preprint study published by Fan Wu et al. ADE by SARS-CoV-2 has been detected from severely affected elderly patients plasma with high titers of SARS-CoV-2 spike protein-specific antibodies via FcγRII cellular receptor; a similar kind of result was obtained when ADE was shown to be mediated by S(spike) protein-specific antibodies in SARS55; furthermore, in a study, anti-nucleocapsid antibody of SARS-CoV was found to be associated with severe cases of illness128; these results offer insights into the possible role of ADE where N-specific antibodies may not be neutralizing leading to more severe disease outcomes.
7 ADE Risk Factors in COVID-19
Antibody-dependent enhancement (ADE) might be one of the causes behind worsening in the severity of symptoms, and this process might have implications for Convalescent therapy used for patients. Due to epitope heterogeneity, there may be a chance that prior exposure to SARS-CoV-2 or other coronaviruses may trigger ADE 129,130. One such observation has been made in the study, which indicated the cross-reactivity of antibodies for previous coronaviruses endemic among the human population 131, and recent studies have shown the presence of IgG seropositivity for OC43 and NL63 in individuals who have not been exposed to SARS-CoV-2 forming immune responses against SARS-CoV-2 132. Thus, prior exposure to other coronaviruses may be a risk factor for ADE. Glycosylation of antibodies, particularly in the Fc region of IgG, has been extensively studied in health and disease. The Fc region of IgG1 antibodies binds with the FcγRIII receptor through interaction with the hinge region and the CH2 domain 133,134. The interaction of Fc with FcγRIII receptor is significantly influenced by the presence of glycans at the N-glycosylation site in each of the CH2 domains 135. It has been widely studied that the absence of core fucose from N-glycans of antibodies leads to an enhanced ADCC activity that subsequently increases affinity for FcγRIIIa both in vitro and in vivo136,137. Also, the researchers have developed non-fucosylated antibodies that, at lower concentrations was shown to exhibit strong ADCC deploying this glycosylation feature of antibodies138. Such a low level of fucosylation has been shown in S, and RBD-specific IgG antibodies in COVID affected symptomatic patients as compared to either asymptomatic or mildly infected patients mediating strong FcγRIIIa responses139,140.
8 Health Issues Associated with ADE
ADE has been observed to potentially escalate multiple viral infections, such as in the case of respiratory syncytial virus (RSV) 53,141 and measles 142,143. The outcome of ADE has been shown to affect lungs, causing lung injury and cause enhancement of respiratory disease after a respiratory virus infection takes place with symptoms of monocytic infiltration and profusion of eosinophils in the respiratory tract 144; the other outcome observed is a vaccine-associated enhanced respiratory disease (VAERD)143. ADE has been known to be associated with a wider category of infections known as enhanced respiratory disease (ERD), including antibody-based mechanisms such as cytokine cascades and cell-mediated immunopathology145. In non-macrophage tropic respiratory viruses, for instance, RSV and measles, non-neutralizing antibodies have been shown to induce ADE and ERD by the formation of immune complexes that get deposited in the airway tissues and activate cytokine and complement pathways that cause inflammation, airway obstruction, and acute respiratory distress syndrome leading to severe cases of illness141,142,143,146,147; and these are some clinical observations of SARS-CoV-2 similar to RSV and measles in severe cases of COVID-19; inflammatory lung injury by activation of hyperactivation of the complement cascade has been observed prevailing in COVID-19 patients 148,149. These results collectively indicate that complement pathways can be aggressively activated in the lungs of COVID-19 patients, which may attribute to SARS-CoV-2N protein150. In vivo studies in BALB/c mice challenged with non-neutralizing antibodies for Influenza A virus demonstrated increased lung pathology and infiltration of cells into the alveolar air space on challenging with neutralizing antibodies43. SARS-CoV-2 may escape the antibody–virus complex at sub-neutralizing concentrations of antibodies progressing towards replication process that may be abortive without producing viable virus particles or non-abortive, in either case, massive death of immune cells can happen that can result in inflammation cascade and a cytokine storm151. As the surge in COVID-19 cases took place, children and adults have been observed to be infected with SARS-CoV-2 more than those observed in the early phase of the COVID-19 pandemic back in 2019. Children who were thought to be largely spared from SARS-CoV-2 had become more prone to multisystem inflammatory syndrome (MIS-C), a post-COVID-19 disorder, which was first recognized in the UK when children were found to be negative for SARS-CoV-2 but were seropositive, indicates the possibility of past infection; multisystem inflammatory syndrome in infants (MIS-C) and adults (MIS-A) associated with COVID-19 has certain implications such as multiple organ failure and shock152,153, in this context, more further research is required to confirm the basis of ADE in MIS-C.
9 Conclusion and Future Anticipation
According to some previous and present studies, cross-reactive non-neutralizing antibodies appear to hamper virus neutralization. However, extensive research on ADE remains to be done to prove its existence; in vitro and in vivo studies done so far underscore the chances of ADE in Flaviviruses infection. Future studies are required to study the immune system physiology of people before and after vaccination as pre-existing antibodies may provide some correlation with recovery as opposed to worsening of disease that may enlighten the type of antibodies to assess in vaccine studies and differences in the severity of COVID-19 illness and how it manifests in old versus young people. Whether the immune system is producing antibodies to the original viral strain from prior exposure or to the currently infecting viral strain needs to be studied. Thus, uncovering these facts and findings could help explain the largely varying response amongst COVID-19 patients with the disease ranging from mild and symptomless to severe infections requiring hospitalization in some cases that often result in death. ADE could be a potentially new avenue to explore in COVID-related fatality.
10 Suggested Recommendations
Globally, new variants of SARS-CoV-2 have emerged that could potentially give rise to the phenomenon of ADE. It is a combination of several factors that may determine its resurgence but nevertheless could trigger reinfection with an enhanced severity and need for healthcare support. The other members of the beta coronavirus lineage, including SARS-CoV and the Middle East respiratory syndrome (MERS) virus, are already known to infect humans. Hence, it is conceivable that protective antibodies against them could initiate ADE in individuals infected with SARS-CoV-2. Maternally acquired SARS-CoV-2 antibodies bound to mast cells could also trigger ADE in children along with the development of MIS (multisystem inflammatory syndrome) via placental transport of these antibodies. However, in contrast to DENV, SARS, and MERS, CoVs infect predominantly the respiratory epithelium, not macrophages which actually expresses the FcγR receptors. Therefore, the chances of ADE are low in COVID cases, and no ADE has been observed in COVID-19 infection. But not just the virus variants; even vaccines against COVID-19 could initiate ADE response. However, the new vaccine technologies consider these facts at the vaccine design stages for any risk of ADE. These are as listed below:
-
Targeting a SARS-CoV-2 protein epitope for vaccine development that was the least likely to cause ADE, as evident from in silico studies.
-
Evaluating animal-based studies in pre-clinical and phase1 trials for ADE post-vaccination studies. Further to do the same in human clinical trials.
-
Epidemiological surveillances to register cases of ADE in a population
It is, therefore, in the best of everyone’s interest to ramp up the vaccination drive keeping a tab on the vaccinated populations for adverse response and hospitalization. Unvaccinated individuals are the breeding grounds for the emergence of variants of interest (VOI) and variants of concern (VOC), which are more harmful than ADE in terms of the rate of occurrence; this needs coordination between the government and the residents with a public awareness program describing the benefits of vaccines in containing the disease spread in the population. We need to understand that if not facilitating the virus entry, even antibody-mediated elevated effector functions or immune complex formation can also lead to ADE and inflammation. With more innovative algorithms, a vaccine against SARS-CoV-2 generated high neutralizing antibody titers and minimal risk of ADE. With many more vaccines coming up in the market against SARS-CoV-2, the government and regulatory bodies must verify the safety and efficacy of these vaccines for their own population. These necessities the requirement for vaccine bridging trials for the foreign-made vaccines to look for ADE in the native population. Even if rare for COVID-19, the possibilities for ADE poses a theoretical risk and need to be addressed with utmost care.
Data availability
Not applicable.
Code availability
Not applicable.
References
Wrapp D, Wang N, Corbett KS et al (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. bioRxiv. https://doi.org/10.1101/2020.02.11.944462
Matta S, Chopra KK, Arora VK (2020) Morbidity and mortality trends of Covid 19 in top 10 countries. Indian J Tuberc 67:S167–S172. https://doi.org/10.1016/J.IJTB.2020.09.031
Mazumder A, Arora M, Bharadiya V et al (2020) SARS-CoV-2 epidemic in India: epidemiological features and in silico analysis of the effect of interventions. F1000Research 9:315. https://doi.org/10.12688/f1000research.23496.2
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. https://doi.org/10.1056/nejmoa2001316
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Jiang S, Hillyer C, Du L (2020) Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 41:355–359
Lu R, Zhao X, Li J et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Lukassen S, Chua RL, Trefzer T et al (2020) SARS -CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. https://doi.org/10.15252/embj.20105114
Astuti I, Ysrafil (2020) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr 14:407–412. https://doi.org/10.1016/j.dsx.2020.04.020
Lechien JR, Chiesa-Estomba CM, de Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277:2251–2261. https://doi.org/10.1007/s00405-020-05965-1
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E et al (2020) Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 34:101623
He X, Cheng X, Feng X et al (2021) Clinical symptom differences between mild and severe COVID-19 patients in China: a meta-analysis. Front Public Health 8:561264
Subbarao K, Mahanty S (2020) Respiratory virus infections: understanding COVID-19. Immunity 52:905–909
Vos LM, Bruyndonckx R, Zuithoff NPA et al (2020) Lower respiratory tract infection in the community: associations between viral aetiology and illness course. Clin Microbiol Infect 27:96. https://doi.org/10.1016/j.cmi.2020.03.023
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Krammer F (2020) SARS-CoV-2 vaccines in development. Nature 586(7830):516–527. https://doi.org/10.1038/s41586-020-2798-3
Kyriakidis NC, López-Cortés A, González EV et al (2021) SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 6(1):1–17. https://doi.org/10.1038/s41541-021-00292-w
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433
Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19. Accessed 28 Apr 2021
Wang H, Li X, Li T et al (2020) The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis 39:1629–1635
Zhang YZ, Holmes EC (2020) A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 181:223–227. https://doi.org/10.1016/j.cell.2020.03.035
Naqvi AAT, Fatima K, Mohammad T et al (2020) Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis 1866:165878
Hoffmann M, Hofmann-Winkler H, Pöhlmann S (2018) Priming time: how cellular proteases arm coronavirus spike proteins. Activation of viruses by host proteases. Springer International Publishing, Berlin, pp 71–98
To KK, Hung IF, Ip JD et al (2020) COVID-19 reinfection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. https://doi.org/10.1093/CID/CIAA1275
Van Elslande J, Vermeersch P, Vandervoort K et al (2021) Symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection by a phylogenetically distinct strain. Clin Infect Dis 73:354–356. https://doi.org/10.1093/CID/CIAA1330
Prado-Vivar B, Becerra-Wong M, Guadalupe JJ et al (2021) A case of SARS-CoV-2 reinfection in Ecuador. Lancet Infect Dis 21:e142. https://doi.org/10.1016/S1473-3099(20)30910-5
Tirado SMC, Yoon KJ (2003) Antibody-dependent enhancement of virus infection and disease. Viral Immunol 16:69–86. https://doi.org/10.1089/088282403763635465
Tamura M, Webster RG, Ennis FA (1994) Subtype cross-reactive, infection-enhancing antibody responses to Influenza A viruses. J Virol 68:3499–3504. https://doi.org/10.1128/jvi.68.6.3499-3504.1994
Stettler K, Beltramello M, Espinosa DA et al (2016) Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. https://doi.org/10.1126/science.aaf8505
Halstead SB, O’rourke EJ (1977) Dengue viruses and mononuclear phagocytes I. Infection enhancement by non-neutralizing antibody. J Exp Med 146:201–217. https://doi.org/10.1084/jem.146.1.201
Du L, He Y, Zhou Y et al (2009) The spike protein of SARS-CoV - A target for vaccine and therapeutic development. Nat Rev Microbiol 7:226–236
Burton DR (2002) Antibodies, viruses and vaccines. Nat Rev Immunol 2:706–713
Ryu W-S (2017) Virus life cycle. Molecular virology of human pathogenic viruses. Elsevier, Amsterdam, pp 31–45
Mandel B (1978) Neutralization of animal viruses. Adv Virus Res 23:205–268. https://doi.org/10.1016/S0065-3527(08)60101-3
Klasse PJ (2014) Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol 2014:1–24. https://doi.org/10.1155/2014/157895
Klasse PJ, Sattentau QJ (2002) Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 83:2091–2108
Daniels CA (1975) Mechanisms of viral neutralization. Viral immunology and immunopathology. Elsevier, Amsterdam, pp 79–97
Taylor A, Foo SS, Bruzzone R et al (2015) Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev 268:340–364
Kulkarni R (2020) Antibody-dependent enhancement of viral infections. Dynamics of immune activation in viral diseases. Springer, Singapore, pp 9–41
Porterfield JS (1986) Antibody-dependent enhancement of viral infectivity. Adv Virus Res 31:335–355. https://doi.org/10.1016/S0065-3527(08)60268-7
Felsenstein S, Hedrich CM (2020) COVID-19 in children and young people. Lancet Rheumatol 2:e514–e516
Wen J, Cheng Y, Ling R et al (2020) Antibody-dependent enhancement of coronavirus. Int J Infect Dis 100:483–489
Winarski KL, Tang J, Klenow L et al (2019) Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc Natl Acad Sci USA 116:15194–15199. https://doi.org/10.1073/pnas.1821317116
von Kietzell K, Pozzuto T, Heilbronn R et al (2014) Antibody-mediated enhancement of parvovirus B19 uptake into endothelial cells mediated by a receptor for complement factor C1q. J Virol 88:8102–8115. https://doi.org/10.1128/jvi.00649-14
Arvin AM, Fink K, Schmid MA et al (2020) A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584:353–363
Wan Y, Shang J, Sun S et al (2019) Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol 94:2015–2034. https://doi.org/10.1128/jvi.02015-19
Khandia R, Munjal A, Dhama K et al (2018) Modulation of Dengue/Zika Virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in Zika Virus infection. Front Immunol 9:1
Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467. https://doi.org/10.1016/S0065-3527(03)60011-4
Graham BS (2020) Rapid COVID-19 vaccine development. Science 368:945–946. https://doi.org/10.1126/science.abb8923
Cloutier M, Nandi M, Ihsan AU et al (2020) ADE and hyperinflammation in SARS-CoV2 infection- comparison with dengue hemorrhagic fever and feline infectious peritonitis. Cytokine 136:155256
Ou J, Zhou Z, Dai R et al (2021) V367F mutation in SARS-CoV-2 spike RBD emerging during the early transmission phase enhances viral infectivity through increased human ACE2 receptor binding affinity. J Virol. https://doi.org/10.1128/JVI.00617-21
Jin X, Xu K, Jiang P et al (2020) Virus strain from a mild COVID-19 patient in Hangzhou represents a new trend in SARS-CoV-2 evolution potentially related to Furin cleavage site. Emerg Microbes Infect 9:1474–1488. https://doi.org/10.1080/22221751.2020.1781551
Kim HW, Canchola JG, Brandt CD et al (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. https://doi.org/10.1093/oxfordjournals.aje.a120955
Kapikian AZ, Mitchell RH, Chanock RM et al (1969) An epidemiologic study of altered clinical reactivity to Respiratory Syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. https://doi.org/10.1093/oxfordjournals.aje.a120954
Wang SF, Tseng SP, Yen CH et al (2014) Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun 451:208–214. https://doi.org/10.1016/j.bbrc.2014.07.090
von Holle TA, Anthony Moody M (2019) Influenza and antibody-dependent cellular cytotoxicity. Front Immunol 10:1457
Pincetic A, Bournazos S, Dilillo DJ et al (2014) Type i and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 15:707–716
Li M, Zhao L, Zhang C et al (2018) Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS One. https://doi.org/10.1371/journal.pone.0200478
Kou Z, Quinn M, Chen H et al (2008) Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol 80:134–146. https://doi.org/10.1002/jmv.21051
Sun P, Bauza K, Pal S et al (2011) Infection and activation of human peripheral blood monocytes by dengue viruses through the mechanism of antibody-dependent enhancement. Virology 421:245–252. https://doi.org/10.1016/j.virol.2011.08.026
Flipse J, Diosa-Toro MA, Hoornweg TE et al (2016) Antibody-dependent enhancement of dengue virus infection in primary human macrophages; balancing higher fusion against antiviral responses. Sci Rep 6:31–50. https://doi.org/10.1038/srep29201
Hamzeh-Cognasse H, Damien P, Chabert A et al (2015) Platelets and infections - complex interactions with bacteria. Front Immunol 6:82
Barnes N, Gavin AL, Tan PS et al (2002) FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 16:379–389. https://doi.org/10.1016/S1074-7613(02)00287-X
Hulett MD, Hogarth PM (1998) The second and third extracellular domains of FcγRI (CD64) confer the unique high affinity binding of IgG2a. Mol Immunol 35:989–996. https://doi.org/10.1016/S0161-5890(98)00069-8
Dunkelberger JR, Song WC (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. https://doi.org/10.1038/cr.2009.139
Dustin ML (2016) Complement receptors in myeloid cell adhesion and phagocytosis. In: Myeloid cells in health and disease. American Society of Microbiology, pp 429–445
Takada A, Feldmann H, Ksiazek TG, Kawaoka Y (2003) Antibody-dependent enhancement of Ebola virus infection. J Virol 77:7539–7544. https://doi.org/10.1128/jvi.77.13.7539-7544.2003
Eggleton P, Reid KBM (1998) C1q - how many functions? How many receptors? Trends Cell Biol 8:428–431. https://doi.org/10.1016/S0962-8924(98)01373-7
Nicholson-Weller A, Klickstein LB (1999) C1q-binding proteins and C1q receptors. Curr Opin Immunol 11:42–46. https://doi.org/10.1016/S0952-7915(99)80008-9
French MA (2019) Antibody-mediated control of HIV-1 infection through an alternative pathway. AIDS 33:1961–1966. https://doi.org/10.1097/QAD.0000000000002313
Hawkes RA, Lafferty KJ (1967) The enhancement of virus infectivity by antibody. Virology 33:250–261. https://doi.org/10.1016/0042-6822(67)90144-4
Mazzoli S, Lopalco L, Salvi A et al (1999) Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis 180:871–875. https://doi.org/10.1086/314934
Jane Cardosa M, Porterfield JS, Gordon S (1983) Complement receptor mediates enhanced flavivirus replication in macrophages. J Exp Med 158:258–263. https://doi.org/10.1084/jem.158.1.258
Payne S (2017) Immunity and resistance to viruses. Viruses. Elsevier, Amsterdam, pp 61–71
VanBlargan LA, Goo L, Pierson TC (2016) Deconstructing the antiviral neutralizing-antibody response: implications for vaccine development and immunity. Microbiol Mol Biol Rev 80:989–1010. https://doi.org/10.1128/mmbr.00024-15
Dowd KA, Jost CA, Durbin AP et al (2011) A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1002111
Dowd KA, Jost CA, Durbin AP et al (2011) A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog 7:1002111. https://doi.org/10.1371/journal.ppat.1002111
Mok DZL, Chan KR (2020) The effects of pre-existing antibodies on live-attenuated viral vaccines. Viruses 12:520
Adair RA, Roulstone V, Scott KJ et al (2012) Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3003578
Hodgins DC, Shewen PE (2012) Vaccination of neonates: problem and issues. Vaccine 30:1541–1559
Guzman MG, Vazquez S (2010) The complexity of antibody-dependent enhancement of dengue virus infection. Viruses 2:2649–2662. https://doi.org/10.3390/v2122649
Pinto D, Park YJ, Beltramello M et al (2020) Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583:290–295. https://doi.org/10.1038/s41586-020-2349-y
Yuan M, Wu NC, Zhu X et al (2020) A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 368:630–633. https://doi.org/10.1126/science.abb7269
McAndrews KM, Dowlatshahi DP, Dai J et al (2020) Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight. https://doi.org/10.1172/JCI.INSIGHT.142386
Rogers TF, Zhao F, Huang D et al (2020) Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369:956–963. https://doi.org/10.1126/science.abc7520
Long QX, Liu BZ, Deng HJ et al (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. https://doi.org/10.1038/s41591-020-0897-1
Fierz W, Walz B (2020) Antibody dependent enhancement due to original antigenic sin and the development of SARS. Front Immunol 11:1120. https://doi.org/10.3389/fimmu.2020.01120
Davenport FM, Hennessy AV, Francis T (1953) Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med 98:641–656. https://doi.org/10.1084/jem.98.6.641
Henry C, Palm AKE, Krammer F, Wilson PC (2018) From original antigenic sin to the universal influenza virus vaccine. Trends Immunol 39:70–79
Corti D, Lanzavecchia A (2013) Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31:705–742
Hawkes RA (1964) Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci 42:465–482. https://doi.org/10.1038/icb.1964.44
Camargo FDA, Adimy M, Esteva L et al (2021) Modeling the relationship between antibody-dependent enhancement and disease severity in secondary dengue infection. Bull Math Biol. https://doi.org/10.1007/s11538-021-00919-y
Krilov LR, Anderson LJ, Marcoux L et al (1989) Antibody-mediated enhancement of respiratory syncytial virus infection in two monocyte/macrophage cell lines. J Infect Dis 160:777–782. https://doi.org/10.1093/infdis/160.5.777
Willey S, Aasa-Chapman MMI, O’Farrell S et al (2011) Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology 8:16. https://doi.org/10.1186/1742-4690-8-16
Sauter P, Hober D (2009) Mechanisms and results of the antibody-dependent enhancement of viral infections and role in the pathogenesis of coxsackievirus B-induced diseases. Microbes Infect 11:443–451
Hohdatsu T, Yamada M, Tominaga R et al (1998) Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J Vet Med Sci 60:49–55. https://doi.org/10.1292/jvms.60.49
Liu L, Wei Q, Lin Q et al (2019) Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. https://doi.org/10.1172/JCI.INSIGHT.123158
Luo F, Liao FL, Wang H et al (2018) Evaluation of antibody-dependent enhancement of SARS-cov infection in rhesus macaques immunized with an inactivated SARS-CoV vaccine. Virol Sin 33:201–204
Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS | HKMJ. https://www.hkmj.org/abstracts/v22n3%20Suppl%204/25.htm. Accessed 9 Aug 2021
Du L, Zhao G, Yang Y et al (2014) A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in middle east respiratory syndrome coronavirus spike protein. J Virol 88:7045. https://doi.org/10.1128/JVI.00433-14
Schlesinger JJ, Brandriss MW (1981) Antibody-mediated infection of macrophages and macrophage-like cell lines with 17D-yellow fever virus. J Med Virol 8:103–117. https://doi.org/10.1002/JMV.1890080204
Halstead SB, O’rourke EJ (1977) Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741. https://doi.org/10.1038/265739A0
Halstead SB (1979) In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140:527–533. https://doi.org/10.1093/INFDIS/140.4.527
Homsy J, Meyer M, Tateno M et al (1989) The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 244:1357–1360. https://doi.org/10.1126/SCIENCE.2786647
Robinson WE, Montefiori D, Mitchell W (1988) Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet 1:790–794. https://doi.org/10.1016/S0140-6736(88)91657-1
Takeda A, Tuazon CU, Ennis FA (1988) Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science 242:580–583. https://doi.org/10.1126/SCIENCE.2972065
Yao JS, Kariwa H, Takashima I et al (1992) Antibody-dependent enhancement of hantavirus infection in macrophage cell lines. Arch Virol 122:107–118. https://doi.org/10.1007/BF01321121
Takada A, Watanabe S, Okazaki K et al (2001) Infectivity-enhancing antibodies to Ebola virus glycoprotein. J Virol 75:2324–2330. https://doi.org/10.1128/JVI.75.5.2324-2330.2001
Chanas AC, Gould EA, Clegg JC, Varma MG (1982) Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol 58(Pt 1):37–46. https://doi.org/10.1099/0022-1317-58-1-37
Millican D, Porterfield JSP (1982) Relationship between glycoproteins of the viral envelope of bunyaviruses and antibody-dependent plaque enhancement. J Gen Virol 63(Pt 1):233–236. https://doi.org/10.1099/0022-1317-63-1-233
Ochiai H, Kurokawa M, Hayashi K, Niwayama S (1988) Antibody-mediated growth of Influenza A NWS virus in macrophagelike cell line P388D1. J Virol 62:20
Ochiai H, Kurokawa M, Matsui S et al (1992) Infection enhancement of Influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J Med Virol 36:217–221. https://doi.org/10.1002/JMV.1890360312
Weiss RC, Scott FW (1981) Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis 4:175. https://doi.org/10.1016/0147-9571(81)90003-5
King AA, Sands JJ, Porterfield JS (1984) Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1). J Gen Virol 65(Pt 6):1091–1093. https://doi.org/10.1099/0022-1317-65-6-1091
Inada T, Chong KT, Mims CA (1985) Enhancing antibodies, macrophages and virulence in mouse cytomegalovirus infection. J Gen Virol 66(Pt 4):871–878. https://doi.org/10.1099/0022-1317-66-4-871
Baxt B, Mason PW (1995) Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology 207:503–509. https://doi.org/10.1006/VIRO.1995.1110
Jarasch-Althof N, Wiesener N, Schmidtke M et al (2010) Antibody-dependent enhancement of coxsackievirus B3 infection of primary CD19+ B lymphocytes. Viral Immunol 23:369–376. https://doi.org/10.1089/VIM.2010.0018
Halstead SB, Shotwell H, Casals J (1973) Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis 128:15–22. https://doi.org/10.1093/INFDIS/128.1.15
Füst G, Tóth FD, Kiss J et al (1994) Neutralizing and enhancing antibodies measured in complement-restored serum samples from HIV-1-infected individuals correlate with immunosuppression and disease. AIDS 8:603–609. https://doi.org/10.1097/00002030-199405000-00005
Furuyama W, Marzi A, Carmody AB et al (2016) Fcγ-receptor IIa-mediated Src signaling pathway is essential for the antibody-dependent enhancement of ebola virus infection. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1006139
Guzman MG, Alvarez M, Halstead SB (2013) Secondary infection as a risk factor for Dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Adv Virol 158:1445–1459
Kliks SC, Nimmanitya S, Nisalak A, Burke DS (1988) Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38:411–419. https://doi.org/10.4269/ajtmh.1988.38.411
Chan KR, Wang X, Saron WAA et al (2016) Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nat Microbiol 1:1–10. https://doi.org/10.1038/nmicrobiol.2016.164
Saito Y, Moi ML, Takeshita N et al (2016) Japanese encephalitis vaccine-facilitated dengue virus infection-enhancement antibody in adults. BMC Infect Dis 16:578. https://doi.org/10.1186/s12879-016-1873-8
Zhao J, Yuan Q, Wang H et al (2020) Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 71:2027–2034. https://doi.org/10.1093/cid/ciaa344
Tillett RL, Sevinsky JR, Hartley PD et al (2020) Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(20)30764-7
Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS | HKMJ. https://www.hkmj.org/abstracts/v22n3%20Suppl%204/25.htm. Accessed 12 May 2021
Leung DTM, Tam FCH, Chun HM et al (2004) Antibody response of patients with Severe Acute Respiratory Syndrome (SARS) targets the viral nucleocapsid. J Infect Dis 190:379–386. https://doi.org/10.1086/422040
Cegolon L, Pichierri J, Mastrangelo G et al (2020) Hypothesis to explain the severe form of COVID-19 in Northern Italy. BMJ Glob Health 5:e002564
Tetro JA (2020) Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect 22:72–73. https://doi.org/10.1016/j.micinf.2020.02.006
Ladner JT, Henson SN, Boyle AS et al (2020) Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with an endemic human CoV. bioRxiv. https://doi.org/10.1101/2020.07.27.222943
Ma Z, Li P, Ji Y et al (2020) Cross-reactivity towards SARS-CoV-2: the potential role of low-pathogenic human coronaviruses. Lancet Microbe 1:e151. https://doi.org/10.1016/s2666-5247(20)30098-7
Radaev S, Motyka S, Fridman WH et al (2001) The structure of a human type III Fcγ receptor in complex with Fc. J Biol Chem 276:16469–16477. https://doi.org/10.1074/jbc.M100350200
Sondermann P, Huber R, Oosthulzen V, Jacob U (2000) The 3.2-Å crystal structure of the human IgG1 Fc fragment-FcγRIII complex. Nature 406:267–273. https://doi.org/10.1038/35018508
Krapp S, Mimura Y, Jefferis R et al (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol 325:979–989. https://doi.org/10.1016/S0022-2836(02)01250-0
Shields RL, Lai J, Keck R et al (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740. https://doi.org/10.1074/jbc.M202069200
Shinkawa T, Nakamura K, Yamane N et al (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473. https://doi.org/10.1074/jbc.M210665200
Yamane-Ohnuki N, Satoh M (2009) Production of therapeutic antibodies with controlled fucosylation. MAbs 1:230–236
Chakraborty S, Edwards K, Buzzanco AS et al (2020) Symptomatic SARS-CoV-2 infections display specific IgG Fc structures. medRxiv. https://doi.org/10.1101/2020.05.15.20103341
Larsen MD, de Graaf EL, Sonneveld ME et al (2021) Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. https://doi.org/10.1126/science.abc8378
Graham BS (2016) Vaccines against respiratory syncytial virus: the time has finally come. Vaccine 34:3535–3541. https://doi.org/10.1016/j.vaccine.2016.04.083
Nader PR, Horwitz MS, Rousseau J (1968) Atypical exanthem following exposure to natural measles: eleven cases in children previously inoculated with killed vaccine. J Pediatr 72:22–28. https://doi.org/10.1016/S0022-3476(68)80396-8
Polack FP (2007) Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res 62:111–115
Su S, Du L, Jiang S (2020) Learning from the past: development of safe and effective COVID-19 vaccines. Nat Rev Microbiol 1:211–219
Lee WS, Wheatley AK, Kent SJ, DeKosky BJ (2020) Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 5:1185–1191. https://doi.org/10.1038/s41564-020-00789-5
Polack FP, Teng MN, Collins PL et al (2002) A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 196:859–865. https://doi.org/10.1084/jem.20020781
Polack FP, Hoffman SJ, Crujeiras G, Griffin DE (2003) A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 9:1209–1213. https://doi.org/10.1038/nm918
Gao T, Hu M, Zhang X et al (2020) Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv 2020.03.29.20041962
Gralinski LE, Sheahan TP, Morrison TE et al (2018) Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. https://doi.org/10.1128/mBio.01753-18
Gao T, Hu M, Zhang X et al (2020) Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv 2020.03.29.20041962. https://doi.org/10.1101/2020.03.29.20041962
Nechipurenko YD, Anashkina AA, Matveeva OV (2020) Change of antigenic determinants of SARS-CoV-2 virus S-protein as a possible cause of antibody-dependent enhancement of virus infection and cytokine storm. Biophysics 65:703–709. https://doi.org/10.1134/S0006350920040119
Jiang L, Tang K, Levin M et al (2020) COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 20:e276–e288
Ricke DO (2021) Two different antibody-dependent enhancement (ADE) risks for SARS-CoV-2 antibodies. Front Immunol 12:640093. https://doi.org/10.3389/fimmu.2021.640093
Acknowledgements
Authors acknowledge the support extended by Dr. Balram Bhargava, Secretary Department of Health Research, Ministry of Health and Family Welfare, and Director General, Indian Council of Medical Research (ICMR), New Delhi for providing computational facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajmeriya, S., Kumar, A., Karmakar, S. et al. Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective. J Indian Inst Sci 102, 671–687 (2022). https://doi.org/10.1007/s41745-021-00268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-021-00268-8