Abstract
The Science Granting Councils Initiative (SGCI) in Africa aims to strengthen the capacities of selected science granting councils (SGCs) in sub-Saharan Africa in order to support research and evidence-based policies that will contribute to Africa’s economic and social development. As part of SGCI, a study was conducted in 2021 to investigate strategies that have been adopted by fifteen SGCs participating in SGCI in promoting ethical practice in research and innovation. Data collection for the study was mainly based on a data abstraction form that was completed for each country by an assigned focal person with a background in research ethics. The focal persons relied on various methods including document and website review and interviews with senior officers at the SGCs. The study specifically sought to describe the strategies and activities being implemented by the 15 SGCs in promoting ethics and integrity in research and innovation. The study revealed various strategies that were being implemented by the 15 SGCs aimed at promoting ethics in research and innovation including requiring proof of research ethics committee approval before releasing research funds and the inclusion of ethics questions in the application form for funding. It was observed that some activities and strategies were generic to most SGCs for example the development of general/standard guidelines for the conduct of research in each respective country. Overall, the different SGCs were involved in a broad spectrum of activities aimed at promoting research ethics and this paper presents an opportunity for cross fertilization of ideas. By providing a summary of the various strategies that SGCs are using in promoting ethical conduct of research, it is hoped that this paper will lead to improvements in the ways SGCs provide support and oversight over the research that they fund.
Similar content being viewed by others
Background
National research landscapes consist of several key players including researchers, research institutions, policy makers, research funders, research ethics committees (RECs), research volunteers and others who play different roles in ensuring the ethical and successful implementation of research. The area of research ethics, has been dominated and guided by developments in the medical research field and yet it is applicable to any field. In the past century, society has become sensitive to the potential exploitation and abuse of research volunteers who make sacrifices by agreeing to participate in research and being placed at the risk of harm for the good of society, hence the emphasis on research ethics (Ndebele, 2011). Research ethics can be defined as norms for conduct that distinguish between acceptable and unacceptable behaviour in research and it deals with the rights and wrongs in research, values of science and expected standards of conduct in science. Research ethics is concerned about harms to humans, animals and the environment during the conduct of the research and ethical requirements have been developed to ensure the minimization of the possibility of exploitation and harm (Miller and Boulton 2007; Beauchamp and Childress 2001)These ethical requirements need to be respected by all including research funders.
Research ethics committees (RECs) which may be at national, regional or institutional level, are an important aspect of ensuring ethical research and are tasked with the responsibility of reviewing research proposals before research is implemented. They are also tasked with the responsibility of supervising research after approval to ensure that researchers continue to uphold high ethical standards. RECs are responsible for promoting the application of the four basic principles: respect for persons, beneficence, nonmaleficence and justice (Boulton 2008; Beauchamp and Childress 2001). Among other things, they satisfy themselves that the voluntary consent of all potential participants is obtained, that vulnerable populations are protected and not unnecessarily used in research aimed at benefiting others, that risks and burdens associated with research are minimized, that benefits are maximized and that there is fairness in distributing the burdens and benefits of research.
Kruger et al. (2014) in their contribution to a book on ‘Research Ethics in Africa’ mapped the status of research oversight systems and practices in Africa and reported that research activities in Africa had not automatically been complemented by advances in ‘health research oversight systems and functional ethical review committees. Advancement in human research, requires commensurate ethics review structures and functions in the form of effective and efficient Research Ethics Committees (RECs) as well as supporting policies and regulations. The failure of the research oversight systems across Africa to improve, leaves the African continent and its citizens vulnerable to exploitative and harmful research (Ndebele et al. 2014; Noor 2009). The various challenges that African countries have been and are still facing have been mainly due to poor resource availability and lack of human resource capacity (IJsselmuiden et al. 2007; Kasule et al. 2016). A similar situation has been observed among North-African countries (Marzouk et al. 2014). In response to the gaps cited above, various organizations have stepped in to support research ethics capacity building across Africa. These include the Fogarty International Centre at the National Institutes of Health that has supported the establishment of research ethics training programs and European and Developing Countries Clinical Trial Partnership (EDCTP) that has been directly supporting the strengthening of institutional and national research ethics committees across Africa (Ndebele et al. 2014).
There has been high demand, attention and delivery of diverse learning and ethics capacity building initiatives internationally and in Africa, generally geared towards fostering a better understanding of the ethics of international biomedical and clinical research (Decamp et al. n.d.). This has prompted support to various institutions to establish various health research ethics training initiatives and support research ethics infrastructure. In these research ethics capacity development efforts, most of the focus has been directed at research ethics committees, researchers and research institutions. African Science Granting Councils (SGCs) which serve as national research funders have not been part of these efforts, hence the focus of this paper. SGCs are key national players and strategic partners in promoting ethical conduct of research through implementation of various strategies for example, development of guidelines for the conduct of research in line with relevant research polices. In order to promote ethical conduct of research SGCs need to show commitment towards independent ethics review of all research that they fund by requiring REC approval of proposals before releasing funding, requiring functional RECs at institutional or national level, by offering research ethics training and by sharing various resources that are essential in promoting ethics in research and innovation.
SGCs and ethics in research and innovation
The Science Granting Councils Initiative (SGCI) in Sub-Saharan Africa was established in 2015 and seeks to strengthen capacities of Science Granting Councils (SGCs) in Eastern, Southern, Central and West Africa in order to support research and evidence-based policies that will contribute to economic and social development. The initiative is jointly funded by the United Kingdom’s Foreign, Commonwealth and Development Office (FCDO) [formerly Department for International Development –DFID], Canada’s International Development Research Centre (IDRC), and South Africa’s National Research Foundation (NRF). The objectives of SGCI are to strengthen the ability of participating SGCs to (1) manage research; (2) design and monitor research programmes, and to formulate and implement policies based on the use of robust science, technology and innovation (STI) indicators; (3) support knowledge transfer to the private sector; and; (4) establish partnerships with one another, and with other science system actors. The implementation of these objectives is achieved through regional training courses, individualized on-site training sessions, on-line training, webinars and, collaborative research. At the time of this study, the SGCI was working with 15 SGCs in the following countries; Kenya, Rwanda, Uganda, Tanzania, Ethiopia, Cote d’Ivoire, Burkina Faso, Senegal, Ghana, Zambia, Mozambique, Botswana, Malawi, Namibia and Zimbabwe. The fifteen SGCs were the only ones included in the study being reported in this paper by virtue of their participation in SGCI at the time of the study. The SGCIs principle output include (1) more effective research management practices among Councils, (2) strengthened ability of Councils to design and monitor research programmes, and to formulate and implement policies based on the use of robust science technology and innovation indicators, (3) increased knowledge transfer to the private sector and (4) increasingly coordinated and networked Councils.
As part of the preparatory stages of the first phase of SGCI, a scoping study was conducted among the 15 participating countries between 2012 and 2013 to understand science granting councils’ individual research and capacity strengthening interests and priorities. The study identified Research Ethics as a high priority training need. The study concluded that SGCs in sub Saharan Africa were at a low level of maturity in terms of developing, implementing and enforcing research ethics practices (Mouton et al. 2015). The study that resulted in this paper was implemented as a follow up to this initial scoping study as well as the gaps cited by Kruger et al. (2014) and was implemented in preparation for the second phase of SGCI to understand SGC activities related to ethics in research and innovation. The study also sought to identify best practices being implemented by selected SGCI funding partners as well as other SGCs in Europe and USA in order to recommend them for consideration by the SGCs and SGCI coordinators. In particular, the study sought to address the following key questions:
-
At the national/ Councils level, what are the guidelines for ethics in research and innovation? Do the Councils have ethical guidelines for their grantees? How do such guidelines (where they exist) address the key ethical issues? How are these guidelines aligned (or not) with national research and STI policies?
-
Are there specific ethics issues that are peculiar to collaborative research (collaborations with private sector, cross-country collaborations? How are these issues managed?
-
At the funders level how do the policies and guidelines on ethics affect their relationships with grantees?
-
What can the Councils learn from the funders “good practices” and experiences?
-
At the research level, how are issues of ethics captured and implemented? What are the practical experiences SGCI managers in handling ethical issues.
-
How are the institutional policies on research, innovation, commercialization and valorization facilitated or hindered by practical requirements of ethics and integrity?
-
What are the views, perspectives and experiences of individual researchers and grantees? How do the issues affect their promotions and career opportunities; freedoms and choices on publications, innovation, networks etc.?
While the study focused on both ethics and integrity in research and innovation, this paper only focuses on ethics and findings on integrity are discussed in a separate paper.
Methodology
For data collection, the study mainly relied on a data abstraction form that was designed to gather relevant details on each SGC. The data abstraction form consisted of various sections addressing various issues such as research regulations and policies, national research oversight institutions, SGCs and gender issues, SGCs and open science, SGCs and private sector involvement, SGCs and research ethics issues, SGC guidance documents and calls for proposals, application forms and review checklists, research ethics committees, lessons for other SGCs. Under each subheading, there were various questions that were aimed at collecting relevant information. In order to ensure high quality of data, for each SGC, a focal person was assigned to assist with the collection of data and completion of the data abstraction form. All focal persons had previously received training in research ethics at diploma, masters or doctoral level through various research ethics training programs. In preparation for the study, SGCI coordinators at both AAU and AAS formally wrote to heads and representatives of all the 15 SGCs, informing them about the purpose of this study and requesting them to cooperate by providing all the necessary documents as well as information.
The focal persons extensively reviewed and examined documents such as national policies, research regulations, ethics guidelines, SGC policies, SGC guidelines, SGC application forms, SGC review checklists, SGC annual reports as well as SGCs’ websites. The review was complemented by a review of peer-reviewed literature on ethics and integrity in research as well as international guidance documents. The focal persons held online and telephone interviews with SGC representatives who were mainly senior officers. Individual interviews were also held with a few respondents representing researchers and vulnerable groups in all the 15 countries to gather their views relating to the SGCs and their operations. The study was designed to understand the role of the SGCs in promoting ethics in research and innovation in SSA countries that are participating in SGCI-2 namely Kenya, Rwanda, Uganda, Tanzania, Ethiopia, Côte d’Ivoire, Botswana, Burkina Faso, Senegal, Ghana, Zambia, Mozambique, Malawi, Namibia, and Zimbabwe. The study also looked at practices in Europe, North America as well as non-SGCI countries (South Africa and Nigeria) as points of comparison and sources of lessons for the SGCI.
Findings
The study has shown that there are two main models when it comes to how SGC were constituted; semi-autonomous SGCs and SGCs that operate within government ministries. It has been observed that SGCs that are semi-autonomous in nature have more research ethics related activities compared to those that operate within government ministries or departments. Where SGCs operate as semi-autonomous bodies, they are solely focused on promoting research and innovation and have more control on research related activities due to a more holistic picture of the research and innovation enterprise compared to those, which are based in government departments. Such SGCs give more attention to issues relating to ethics. The availability of officers/ staff or board members with expertise in the areas of Research Ethics also matters. SGCs with officers or board members who have received training in research ethics are more likely to engage in more ethics activities. For example Kenya, Malawi, Tanzania, Uganda and Zimbabwean SGCs have either board members or senior level staff that have received training in research ethics through various training programmes.
The study has revealed various strategies that are being implemented by the 15 SGCs aimed at promoting ethics in research and innovation. It was also observed that some activities and strategies were generic to most SGCs for example the development of general/standard guidelines for the conduct of research in each respective country. The findings for this report have been categorised into five main themes namely; funding to cover operational costs of research ethics committees, regulations and guidelines to promote ethical conduct of research, research ethics training, ethical review and monitoring of research projects, and governance of emergency research. Findings on strategies aimed at promoting the inclusion of women and minorities in research are discussed in a separate paper.
Funding to cover operational costs of research ethics committees: It was observed that only two SGCs (Kenya and Senegal) out of the 15, provided some funding to support the operational costs of running RECs in their respective countries. For 14 of the 15 countries, it was observed that RECs receive some of the funding to cover operational expenses from protocol processing fees that are paid by investigators who submit their studies for review. It was also noted that processing fees differ per REC with some charging a small review fee per protocol (USD50) while some charged above USD$500. In the case of Kenya, it was revealed that the SGC allocated funding to a few RECs through a competitive multi-disciplinary grant application to only eligible institutions. In the case of Senegal funds were provided to the national REC which mainly provided oversight over health/medical research.
Regulations and guidelines to promote ethical conduct of research: It was observed that all 15 countries have either regulations or guidelines on the ethical conduct of research. In three of the 15 countries (Malawi, Uganda, Kenya), guidelines and regulations have been issued by the SGCs. In the rest of the countries, they have been issued either by NRECs or Ministries of Health. Where guidelines and regulations have been issued by other entities, the SGCs expect researchers to follow them even for research funded by the SGCs. For all countries relevant regulations and guidelines were publicly available through the websites. Although general guidelines are available, there are challenges in applying these general guidelines. For example one SGC reported that during the current pandemic of Covid-19, there are no clear guidance to RECs on how their registrations can be terminated if their activities are found not to meet the established standards for independent review of research protocols. Two SGC also reported challenges in resolving conflicts at institutional level for example in REC membership and REC chairperson roles and responsibilities. Some SGC representatives also reported that there was lack of clear terms of reference for committee members and chairpersons in RECs that are embedded in institutions of higher learning.
Research ethics training
It was observed that all 15 SGCs do not provide training to REC members or researchers aimed at sharing knowledge and skills that would promote ethical conduct of research. It was reported that there were gaps in coordinating training at both SGC and REC level. SGC representatives observed that RECs were responsible for training their respective REC members and researchers and that RECs were mandated to ensure that REC members have participated in Research ethics education before they are assigned to review research projects. It was reported that in four of the 15 countries (Kenya, Mozambique, Tanzania and Uganda) there were institutions that were offering research ethics education at certificate, diploma and masters level in order to build leadership in research ethics in Africa.
Ethical Review and Monitoring of research projects
It was observed that all SGCs required REC approvals particularly for projects in the health sector. Only five RECS reported that they insist on REC approval for any human research projects. It was observed that 13 out of 15 SGC do not have procedures for monitoring funded human research activities in their respective countries to ensure compliance with national and international ethics guidelines. Some SGC representatives however opined that the centralization of registration of RECs, implied that the monitoring of research projects is conducted by respective RECs that approved the projects. Many RECs are based in academic institutions or hospitals which carry the whole responsibility of ensuring monitoring of project activities to protect the safety and welfare of research participants. The reliance on RECs for monitoring, mainly covers projects in the health field thereby leaving other non-health projects unmonitored. Funding was reported as a major challenge hindering the implementation of monitoring activities by SCGs despite having relevant personnel responsible to conduct such activities.
Governance of emergency research
It was observed that 14 SGCs did not have guidelines that govern the conduct, reporting and implementation of emergency research. However some SGC representatives indicated that strategies to ensure continuation of research projects during Covid-19 were developed by some RECs. The various strategies that had been implemented by RECs for example included conducting meetings virtually, reliance on other RECS/IRBs in study/projects review, development of guidelines on the conduct of projects, and revision of the review systems by adopting for example a review of concept notes to evaluate if projects require immediate review. The SGC representatives also provided various recommendations on how ethical conduct of research could be promoted during the Covid-19 pandemic for example the need to implement REC electronic review systems to support virtual review of proposals, and the development of emergency guidelines to guide RECs in the review and monitoring of study activities.
Best Practices for promoting ethical conduct of research
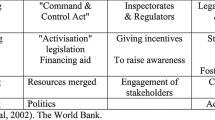
A review of practices by African SGCs, funding partner SGCs as well as other SGCs in Africa, Europe and USA yielded numerous best practices that can be adopted by African SGCs. In Table 1, we present some of these strategies including notes on the approaches that African SGCs may take in promoting ethical conduct of research. Some of the strategies apply at SGC level while some apply at national level.
Discussion
From the review of the 15 SGCs and their roles in promoting research ethics, we have come up with three models on SGC involvement in promoting research ethics as illustrated in Table 2. The first model relates to SGCs that give high priority to research ethics and are directly involved in promoting research ethics. Examples of such SGCs include Malawi and Uganda. The second model describes SGCs that recognize research ethics committees and such SGCs are indirectly involved in promoting research ethics. The majority of SGCs fall into this category in the area of health research as they insist on REC approval for health research projects. The third model, describes SGC that do not give priority to research ethics. Some of the SGCs we reviewed fall into this third model when it comes to human research that does not fall under the health field as they do not require REC approval for such. The second model is viewed as best for Africa as it recognizes the separation of roles between the SGCs and RECs thereby avoiding mission creep.
All 15 countries had general guidelines for the conduct of research and these are authored by SGCs, different authorities including Ministries of Health and Institutional Ethics Committees who have been mandated by their governments to develop ethics regulations and guidelines based on the interpretation of the laws of the countries (De Vries et al. 2017). It was interesting to note that in all 15 countries, regulations and guidelines exist on the conduct of clinical research including procedures and requirements for clinical trial authorization. These regulations and guidelines have been issued by Ministries of Health or national drug regulatory authorities. For non-clinical studies, practical, ethical and regulatory challenges for the proper review and monitoring of activities still exist and need to be addressed. There is less clarity in most SGCs on expectations and their role towards NRECs and institutional RECs. There is also lack of clarity on the SGC roles in development of research ethics guidelines, training of researchers and REC members, monitoring of research that they fund. Clarity is also lacking on the relationship between SGCs and other stakeholders responsible for the conduct of research in countries they are operating. In a world where research is evolving, there is a greater need for clear regulations and guidelines for the conduct of research even in emergency situations. It is recommended that to promote ethical conduct of research that is funded by SGCs, the SGCs should establish appropriate regulatory and ethical guidelines and consolidate with all requirements from institutional RECs. Diverse review procedures and regulations for research have been known to result in REC shopping practices (Kass et al. 2007). As the role of SGCs is to promote the conduct of research, it remains their responsibility to ensure that all research stakeholders actively participate in efforts aimed at promoting ethical conduct of research (World Health Organization, Office of Compliance 2017).
It was also widely evident that many SGC do not provide effective monitoring of the research activities that they fund. It was also evident that the majority of SGCs do not have personnel trained to offer support to researchers that ensures that research volunteers are not adversely treated. Post award regulatory and governance oversight is generally weak in Africa. Post-approval monitoring of research and the documentation of research experiences of volunteers is, arguably a significant aspect of ensuring that study participants safety and welfare is achieved (Love et al. 2020). To this end, monitoring of research activities requires the development of harmonized regulatory requirements that are flexible yet applicable in African settings where the conduct of research has grown over the past decades.
With specific reference to development of leadership in research ethics, there appears to be lack of financial support to enhance skills and knowledge in research ethics among African SGCs. Many SGCS and research institutions in Africa lack well-developed curricular and training structures to build research ethics capacity. Thus, in spite of the significant number of NIH and other grants in Africa, there appears to be limited resources and systems regarding building research ethics leadership and capacities as a way of attaining higher research ethics standards.
Conclusion
SGCs have a crucial role to play in promoting ethical conduct of research and the protection of human subjects in the research that they fund. Their responsibility also extends to the protection of research animals as well as the environment. However, practical issues were widely observed such as weaknesses in ethical review of all research, monitoring the implementation of research projects, development of relevant and up to date research ethics policies and guidelines to regulate the conduct of research. Another general weaknesses that has been identified relates to the lack of support for training of researchers and ethics committees to deal with the complexities of research design, implementation and reporting. SGCs need to create opportunities to engage with ethics experts, regulatory bodies, for example RECS to inform the development of regulatory guidelines or frameworks that are acceptable and suitable for use in their contexts. It is imperative that engagement of relevant stakeholders will unpack important issues and challenges that require further guidance and support through development of relevant guidelines and training. Engagement with stakeholders will generate a community of practice around research ethics and advance the development of ethical benchmarks for conducting research.
SGCs can also develop training programmes that can be consolidated with ethics training being delivered at institutional level. Training of research stakeholders may include online as well as face-to-face components for institutional REC members and researchers. The training ensures that researchers can play an active role by committing to uphold the values of research ethics in their work and their conduct and to adhere to sound scientific practices including scientific rigor. SGC leaders who show commitment to ethical research conduct, can influence institutional conduct. By providing a summary of the various strategies that SGCs can use in promoting ethical conduct of research, it is hoped that this paper will lead to improvements in the ways SGCs provide support and oversight over the research that they fund.
Recommendations
Based on the above overall findings, the following recommendations that specifically relate to ethics in research and innovation, are put forward:
-
1.
African governments should provide SGCs with funds that can be used to support research ethics systems and activities.
-
2.
SGCs should aim to train ethics and regulatory committee members, scientists and policy-makers through annual regional and international workshops, conferences and science/world cafes to engage and create a demand for knowledge, skills and values in order to provide a platform for the development of regulatory guidelines for policy consideration and implementation of research.
-
3.
SGCs from the Anglo phone and Franco phone countries may consider establishing a network of “thought leaders” that will comprise of regulatory officials and ethics committee members to be a forum for the exchange of best practices and, most importantly, to provide ethics leadership in addressing emerging scientific, ethical and regulatory related challenges in Africa. However, this may offer members from various SGCs an opportunity to develop a general set of guidelines that would be tailored for Africa and then further tailored for any country-specific requirement.
-
4.
SGCs need to strengthen their websites to allow for sharing of various resources like guidelines, training materials as well as more general sharing of resources related to research ethics. The SGCs should update their websites to include documents that are relevant for research ethics.
-
5.
The SGCs should require that all national or institutions REC submit a yearly/quarterly report on monitoring reports for their review as part of efforts to promote oversight of clinical research implementation.
-
6.
The SGCs should designate Research Ethics Officers who will be responsible for coordinating ethics issues to liase with all REC administrators on training needs of REC remembers. This can also be utilised as an opportunity for SGC to also learn of new emerging issues in research that require further guidance and development of guidelines.
While the study findings relate to the 15 SGCs that were included in this study, the recommendations would apply to any SGC on the African continent as well as in any other parts of the world.
Data Availability
The data that support the findings of this study are available from the corresponding author, [PN], upon reasonable request.
References
Beauchamp, Tom L., and James F. Childress. 2001. Principles of biomedical ethics. Fifth: Oxford University Press.
Boulton, Mary. 2008. Research ethics. In Research methods for health and social care, ed. J. Neale. 31–45. Palgrave Macmillan.
Decamp, Matthew, Anna Kalbarczyk, Yukari C Manabe, and K. Nelson Sewankambo. n.d. Comment a new vision for bioethics training in global health. The Lancet Global Health 7 (8): e1002–3. https://doi.org/10.1016/S2214-109X(19)30273-6.
de Vries, Jantina, Syntia Nchangwi Munung, Alice Matimba, Sheryl McCurdy, Odile Ouwe Missi Oukem-Boyer, Ciara Staunton, Aminu Yakubu, and Paulina Tindana. 2017. Regulation of genomic and biobanking research in Africa: A content analysis of ethics guidelines, policies and procedures from 22 African countries. BMC Medical Ethics 18 (1): 1–9. https://doi.org/10.1186/s12910-016-0165-6.
IJsselmuiden, C.B., T.C. Nchinda, S. Duale, N.M., Tumwesigye, and D. Serwadda. 2007. Mapping Africa’s advanced public health education capacity: The AfriHealth Project. Bulletin of the World Health Organization 85 (12): 914–922. https://doi.org/10.2471/BLT.07.045526.
Kass, Nancy E., Adnan Ali Hyder, and Ademola Ajuwon, John Appiah-Poku, Nicola Barsdorf, Dya Eldin Elsayed, Mantoa Mokhachane, et al. 2007. The structure and function of research ethics committees in Africa: A case study. Plos Medicine 4 (1). https://doi.org/10.1371/journal.pmed.0040003.
Kasule, Mary, Douglas Wassenaar, Carel IJsselmuiden, and Boitumelo Mokgatla. 2016. Improving review quality and efficiency of research ethics committees to enhance public health practice in Africa. In Public health ethics: Cases spanning the globe. Ed. Drue H. Barrett, Leonard W. Ortmann, Angus Dawson, Carla Saenz, Andreas Reis, and Gail Bolan. 310-314. Springer Open. https://doi.org/10.1007/978-3-319-23847-0.
Kruger, Mariana, Paul Ndebele, and Lyn Horn. 2014. Research ethics: A resource for research ethics committees. https://doi.org/10.1017/CBO9781107415324.004.
Love, Sharon B., Victoria Yorke-Edwards, Sarah Lensen, and Matthew R. Sydes. 2020. Monitoring in practice - how are UK academic clinical trials monitored? A survey. Trials 21 (1): 1–9. https://doi.org/10.1186/s13063-019-3976-1.
Marzouk, Diaa, Wafaa Abd El Aal, Azza Saleh, Hany Sleem, Meriem Khyatti, Loubna Mazini, Kari Hemminki, and Wagida A. Anwar. 2014. Overview on health research ethics in Egypt and North Africa. European Journal of Public Health 24 (Suppl.1): 87–91. https://doi.org/10.1093/eurpub/cku110.
Miller, Tina. 2007. Changing constructions of informed consent: Qualitative research and complex social worlds. Social Science and Medicine 65 (11): 2199–2211. https://doi.org/10.1016/j.socscimed.2007.08.009.
Mouton, Johann, Jacques Gaillard, and Milandré van Lill. 2015. Functions of science granting councils in Sub-Saharan Africa. In Knowledge production and contradictory functions in African higher education. Ed. Nico Cloete, Peter Maassen and Tracy Bailey. 148-170. African Minds.
Ndebele, 2011, Research ethics. In The Sage handbook of health care ethics, ed. R. Chadwick, Henk ten Have and E. Meslin, 306–325. London: Sage Publications.
Ndebele, Paul, Douglas Wassenaar, Solomon Benatar, Theodore Fleischer, Mariana Kruger, and Clement Adebamowo. 2014. Review of NIH Fogarty-Funded programs 2000-2012. https://doi.org/10.1525/jer.2014.9.2.24.research.
Noor, Ramadhani Abdallah. 2009. Health research oversight in Africa. Acta Tropica 112 (Suppl. 1): 63–70. https://doi.org/10.1016/j.actatropica.2009.08.019.
World Health Organization, Office of Compliance, Risk Management and Ethics. 2017. Code of Conduct for Responsible Research, no. November: 26. https://www.who.int/about/ethics/code-of-conduct-for-responsible-research.
Acknowledgements
The work leading to this paper was commissioned by Association of African Universities (AAU) and African Academy of Sciences (AAS) as part of the Science Granting Council Initiative (SGCI). The SGCI is jointly funded by Canada’s International Development Research Centre (IDRC), South Africa’s National Research Foundation (NRF), the United Kingdom’s Department for International Development (DFID) and the Swedish International Development Cooperation Agency (Sida). Authors also acknowledge the support of the 15 SGCs who cooperated and provided data upon request, as well as the various individuals who contributed in various ways including the collection of data in the 15 SGCI countries.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Paul Ndebele and Zivai Nenguke. The first draft of the manuscript was written by Paul Ndebele and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors do not have any competing interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ndebele, P., Nenguke, Z., Mtande, T. et al. The role of science granting councils in promoting ethics in research and innovation: strategies used by selected African SGCs in promoting ethics in research and innovation. International Journal of Ethics Education 8, 373–387 (2023). https://doi.org/10.1007/s40889-023-00169-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40889-023-00169-7