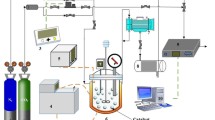
Abstract
Owing to the high Na/V molar ratio of Na3VO4 solution, traditional precipitation of vanadium will consume more H2SO4 and discharge ammonia–nitrogen wastewater with more Na2SO4. In present work, a new process was proposed, in which CO2 was employed to carbonate Na3VO4 solution with simultaneous recovery of NaHCO3, followed by NH4VO3 precipitation and solution recycling. Carbonation kinetics of Na3VO4 solution under both atmospheric and high pressures were studied. Under atmospheric pressure, the solution pH first decreased rapidly, slowly, rapidly again with carbonation time, and finally stabilized at pH 7.3; the formation of CO32− and its conversion to HCO3− mainly occurred and the CO2 absorption rates of different vanadium concentrations were almost the same (about 0.026 mol·m−3·s−1) at pH of 9.0‒9.6. Under high pressure, the reaction rates at early stage increased with CO2 pressure and vanadium concentration, and stirring speed had the most significant effect on reaction rate with the highest CO2 absorption rate of 1.73 mol·m−3·s−1 at 550 rpm. The average CO2 utilization efficiency was ~ 90% with NaHCO3 precipitation of 42.3% at 70 g·L−1 V under 1 MPa of CO2 pressure, while the average CO2 utilization efficiency was ~ 35% under atmospheric pressure. Finally, the reaction rate equation of carbonation under high-pressure was deduced.
Graphical Abstract












Similar content being viewed by others
References
Lee J-c, Kim E-y, Chung KW et al (2021) A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production. J Mater Res Technol 12:343–364
Petranikova M, Tkaczyk AH, Bartl A et al (2020) Vanadium sustainability in the context of innovative recycling and sourcing development. Waste Manage 113:521–544
Gao W, Sun Z, Cao H et al (2020) Economic evaluation of typical metal production process: A case study of vanadium oxide production in China. J Cleaner Prod 256:120217
Zhang X, Meng F, Sun L et al (2022) Influence of several phosphate-containing additives on the stability and electrochemical behavior of positive electrolytes for vanadium redox flow battery. Energies 15:7829
Yi Y-H, Hu S, You J-X et al (2022) Vanadium recovery from Na2SO4-added V-Ti magnetite concentrate via grate-kiln process. T Nonferr Metal Soc 32:2019–2032
Ma J, Li W, Fu G et al (2022) Effect of roasting characteristics on the alkali fusion behavior and mechanism of melting titanium slag. J Sustain Metall 8:1381–1391
Ying Z, Huo M, Wu G et al (2021) Recovery of vanadium and chromium from leaching solution of sodium roasting vanadium slag by stepwise separation using amide and EHEHPA. Sep Purif Technol 269:118741
Zhang X, Meng F, Zhu Z et al (2022) A novel process to prepare high-purity vanadyl sulfate electrolyte from leach liquor of sodium-roasted vanadium slag. Hydrometallurgy 208:105805
Wu K, Wang Y, Wang X et al (2018) Co-extraction of vanadium and chromium from high chromium containing vanadium slag by low-pressure liquid phase oxidation method. J Cleaner Prod 203:873–884
Binnemans K, Jones PT, Manjón Fernández Á et al (2020) Hydrometallurgical processes for the recovery of metals from steel industry by-products: a critical review. J Sustain Metall 6:505–540
Li M, Du H, Zheng S et al (2017) Extraction of vanadium from vanadium slag via non-salt roasting and ammonium oxalate leaching. JOM 69:1970–1975
Chen J-y (2005) Handbook of hydrometallurgy. Metallurgical Industry Press, Beijing
Zhang Y-m, Wang L-n, Chen D-s et al (2018) A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite. Int J Miner Metall Mater 25:131–144
Meng F, Cao L, Yang H et al (2021) Determination and analysis of solubility of Na3VO4 in aqueous NaOH solutions containing Na2CO3, NaAlO2, or Na2SiO3 at 298.15–353.15 K. J Chem Eng Data 66:1574–1581
Wang Z-h, Zheng S-l, Wang S-n et al (2014) Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies. T Nonferr Metal Soc 24:1273–1288
Qu J, Zhang T, Niu L et al (2021) A novel technology to prepare sodium vanadate from V-Cr-bearing reducing slag. Russ J Non-Ferrous Met 62:1–9
Chai Z-l, Wang Y-c, Luan M-l et al (1987) Study on the separation of vanadium from the leaching solution of sodium vanadium slag. Iron Steel Vanadium Titanium 12:33–37
Long S-s, Zhang G-F, Feng Q-M et al (2010) Desiliconisation of alkaline leaching solution of roasted stone coal with carbonation method. T Nonferr Metal Soc 20:s132–s135
Tillmanns E, Baur W (1971) On the crystal chemistry of salt hydrates. VII. The crystal structures of pseudo trisodium orthoarsenate dodecahydrate and the isomorphous phosphate and vanadate salts. Acta Crystallogr Sec B 27:2124–2132
Wang P, Anderko A, Young RD (2002) A speciation-based model for mixed-solvent electrolyte systems. Fluid Phase Equilib 203:141–176
McCann N, Wagner M, Hasse H (2013) A thermodynamic model for vanadate in aqueous solution–equilibria and reaction enthalpies. Dalton Trans 42:2622–2628
Gumerova NI, Rompel A (2020) Polyoxometalates in solution: speciation under spotlight. Chem Soc Rev 49:7568–7601
Wang T-g, Li Z-h (2005) Preliminary study on carbonation kinetics of aqueous sodium chromate. Chem Eng (China) 33:39–42
Yoo M, Han S-J, Wee J-H (2013) Carbon dioxide capture capacity of sodium hydroxide aqueous solution. J Environ Manage 114:512–519
Javed K, Mahmud T, Purba E (2010) The CO2 capture performance of a high-intensity vortex spray scrubber. Chem Eng J 162:448–456
Zhong L-C, Zhou H, Wang L et al (2015) Behavior of bottom injection bubbles in liquid under elevated pressures. J Northeastern Univ (Natural Science) 36:388
Zhang Z, Lu G, Chen Y et al (2020) Alumina extraction from kaolinite via calcification-carbonation process. Russ J Non-Ferrous Met 61:248–256
Gondal S, Asif N, Svendsen HF et al (2015) Kinetics of the absorption of carbon dioxide into aqueous hydroxides of lithium, sodium and potassium and blends of hydroxides and carbonates. Chem Eng Sci 123:487–499
Dalian Research and Design Institute of Chemical Industry (2003) Soda Ash Engineering. Chemical Industry Press, Beijing
Baes CF, Messmer RE (1976) The hydrolysis of cations. John Wiley & Sons, New York
Acknowledgements
This work was supported by National Key Research and Development Program of China (2018YFC1900500), Strategic Priority Research Program of the Chinese Academy of Sciences (XDC04010100), Special Project for Transformation of Major Technological Achievements in Hebei Province (19044012Z), President Fund of China Institute of Standardization (542022Y-9371), and Province Key Research and Development Program of Hebei (20374105D).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no confict of interest.
Additional information
The contributing editor for this article was Mansoor Barati.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chai, X., Meng, FC., Zhang, Xl. et al. Carbonation of Na3VO4 Solution with CO2 for Recovery of NaHCO3 and NH4VO3: Kinetic Analysis of Carbonation Process. J. Sustain. Metall. 9, 588–598 (2023). https://doi.org/10.1007/s40831-023-00674-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00674-5