Abstract
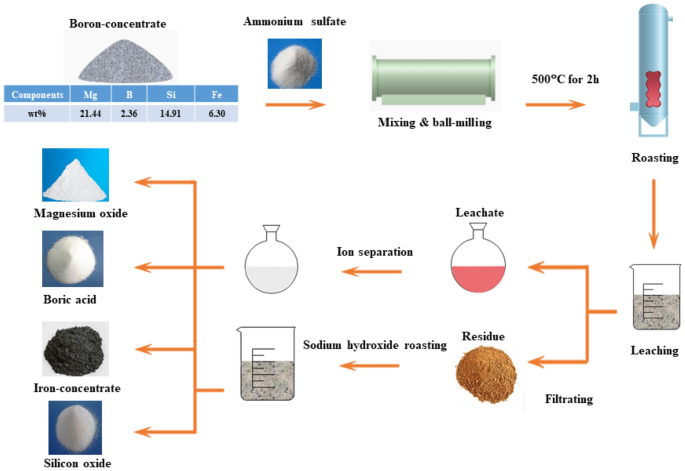
A new eco-friendly process to comprehensively utilize boron-concentrate is proposed in this paper. In this process, Iron-concentrate, silicon dioxide, magnesium oxide, and boric acid could be produced from boron-concentrate. The highlight of this process was minimal waste discharge during the whole process. Iron removal was a key step to ensure the quality of boric acid and magnesium oxide. The introduced goethite precipitation method could remove iron to a very low level. With the addition of 3 cm3 H2O2 (5 vol. %) as an oxidizing agent, more than 99% of iron was removed from the leaching liquor. After the removal of iron, the resulting liquor was used to prepare magnesium precipitate. And the optimal preparation conditions were: the temperature of 30 °C and precipitation time of 60 min, ammonium- magnesium ratio of 1.5, the magnesium ion concentration of 34.8 g·L−1. Under those conditions, the magnesium precipitation efficiency was up to 98.5%. Magnesium oxide was obtained by roasting magnesium precipitate at 550 °C for 2 h. Then the mixed crystals of ammonium sulfate and boric acid were obtained by evaporating the liquor after iron removing and magnesium precipitating. Boric acid was separated from ammonium sulfate by dissolving the mixed crystals in ethanol solution.
Graphical Abstract













Similar content being viewed by others
References
Aghili S, Aghili S, Panjepour M, Panjepour M, Meratian M, Meratian M (2018) Kinetic analysis of formation of boron trioxide from thermal decomposition of boric acid under non-isothermal conditions. J Therm Anal Calorim 131(3):2443–2455
Cao J, Zhao B, Liu S, Zhang M, Lv B (1996) Processing of ascharite-magnetite syngenetic mineral by sulfuric acid(II) phase diagrams of H3bo3-Mgso4-Mgcl2-H2o system at 25°C and 100°C and their application. J Chem Ind Eng (China) 47(4):454–460
Chang Y, Zhai X, Li B, Yan Fu (2010) Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 101(1):84–87
Jiayong C, Jiayong SY, Shuqiu HL, Huizhou XM (1992) New mixed solvent systems for the extraction and separation of ferric iron in sulphate solutions. Hydrometallurgy 30(1–3):401–416
Davey PT, Scott TR (1976) Removal of iron from leach liquors by the "Goethite" process. Hydrometallurgy 2(1):25–33
Duan H, Lv X, Ning Z, Zhai Y (2011) Boron extraction from boron-concentrate ore by ammonium sulfate roasting method. J Northeastern Univ (Natural Science) 32(12):1724–1728
Fu X-J, Chu M-S, Gao L-H, Liu Z-G (2018) Stepwise recovery of magnesium from low-grade ludwigite ore based on innovative and clean technological route. Trans Nonferrous Metal Soc China 28(11):2383–2394
Gao P, Li G, Xiaotian Gu, Han Y (2020) Reduction kinetics and microscopic properties transformation of boron-bearing iron concentrate–carbon-mixed pellets. Miner Process Extr Metall Rev 41(3):162–170
Hu, Q. The Production and Application of Magnesium Compounds, Chemical Industry Publication, 2004. P 11–31.
Kaksonen AH, Morris C, Hilario F, Rea SM, Li J, Usher KM, Wylie J, Ginige MP, Cheng KY, du Plessis C (2014) Iron oxidation and jarosite precipitation in a two-stage airlift bioreactor. Hydrometallurgy 150:227–235
Leussing DL, Kolthoff IM (1953) The solubility product of ferrous hydroxide and the ionization of the aquo-ferrous ion. J Am Chem Soc 75(10):2476–2479
Li G, Liang B, Rao M, Zhang Y, Jiang T (2014) An innovative process for extracting boron and simultaneous recovering metallic iron from ludwigite ore. Miner Eng 56:57–60
Liang B, Li G, Rao M, Peng Z, Zhang Y, Jiang T (2017) Water leaching of boron from soda-ash-activated ludwigite ore. Hydrometallurgy 167:101–106
Liu F, Zhou J, Jin T, Zhang S, Liu L (2016) Effect of calcium oxide on the efficiency of ferrous ion oxidation and total iron precipitation during ferrous ion oxidation in simulated acid mine drainage treatment with inoculation of acidithiobacillus ferrooxidans. Water Sci Technol 73(6):1442–1453
Liu S, Cui C, Zhang X (1998) Pyrometallurgical separation of boron from iron in ludwigite ore. ISIJ Int 38(10):1077–1079
Sheng Y, Kaley B, Bibby K, Grettenberger C, Macalady JL, Wang G, Burgos WD (2017) Bioreactors for low-Ph iron(Ii) oxidation remove considerable amounts of total iron. RSC Advances 7(57):35962–35972
Sutradhar N, Sinhamahapatra A, Roy B, Bajaj HC, Mukhopadhyay I, Panda AB (2011) Preparation of Mgo nano-rods with strong catalytic activity Via hydrated basic magnesium carbonates. Mater Res Bull 46(11):2163–2167
Wang G, Wang J, Ding Y, Ma S, Xue Q (2012) New separation method of boron and iron from ludwigite based on carbon bearing pellet reduction and melting technology. ISIJ Int 52(1):45–51
Wang Y, Li Z, Demopoulos GP (2008) Controlled precipitation of nesquehonite (Mgco3·3h2o) by the reaction of Mgcl2 with (Nh4)2co3. J Cryst Growth 310(6):1220–1227
Wang Z, Chu M, Li Z, Tang J, Zhao Q, Xue X (2011) Fundamental study on application of gas-based shaft furnace direct reduction process to high efficiency and clean utilization of paigeite. Adv Mater Res 233–235:753–758
Wu Y, Pan X-L, Han Y-J, Hai-yan Yu (2019) Dissolution kinetics and removal mechanism of kaolinite in diasporic bauxite in alkali solution at atmospheric pressure. Trans Nonferrous Meta Soc China 29(12):2627–2637
Wu Y, Pan X, Li Q, Haiyan Yu (2020) Crystallization and phase transition of tobermorite synthesized by hydrothermal reaction from dicalcium silicate. Int J Appl Ceram Technol 17(3):1213–1223
Fu XJ, Zhao JQ, Chen SY, Liu ZG, Guo TL, Chu MS (2015) Comprehensive utilization of ludwigite ore based on metallizing reduction and magnetic separation. J Iron Steel Res Int 22(8):672–680
Xiong F-Q, Gui W-H, Yang C-H (2012) Integrated prediction model of iron concentration in goethite method to remove iron process. Kongzhi yu Juece/Control and Decision 27(3):329–342
Yang J, Zhang X-H, Tian Z-H, Xiao-Yun Xu, Liu J (2014) Determination of boron in silica refractory by acid-base titration. Yejin Fenxi/Metallurgical Analysis 34(4):78–80
Ye Y, Lv B (1996) Processing of ascharite-magnetite syngenetic mineral by sulfuric acid(I)—digestion retardation of magnetite and it’s mechanism. J Chem Ind Eng China 47(4):447–453
Zhang C, Fang Li, Liao H, Cheng W, Cheng F (2018) Crystallization of Mgco3·3h2o in Mg(Oh)2-Co2-H2o system. Inorg Chem Ind 50(6):36–41
Zhang X, Li GH, You JX, Wang J, Luo J, Duan JY, Zhang T, Peng ZW, Rao MJ, Jiang T (2019) Extraction of boron from ludwigite ore: mechanism of soda-ash roasting of lizardite and szaibelyite. Minerals 9(9):533
Zhang X, Lang J, Cui C, Liu S (1995) Comprehensive utilization of low grade ludwigite ore during blast furnace smelting. Kang T’ieh/Iron and Steel (Peking) 30(12):9–11
Zhu Z, You J, Zhang X, Li G, Wang J, Luo J, Rao M, Peng Z, Jiang T (2020) Recycling excessive alkali from reductive soda ash roasted ludwigite ore: toward a zero-waste approach. ACS Sustain Chem Eng 8(13):5317–5327
Acknowledgements
The authors appreciate financial support by the Natural Science Foundation of Chongqing (No. cstc2018jcyjAX0792.s) and the Fundamental Funds for the Central Universities (No. 2020CDJ-LHSS-010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Hiromichi Takebe.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, H., Wang, C., Fang, Y. et al. Comprehensive Utilization of Boron-Concentrate by Hydrometallurgy. J. Sustain. Metall. 7, 244–255 (2021). https://doi.org/10.1007/s40831-020-00328-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00328-w




