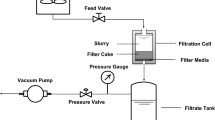
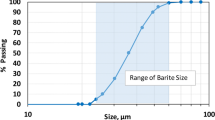
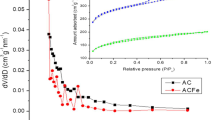
Abstract
Basic oxygen steelmaking (BOS) filter cake has been found to undergo a self-sintering process, improving its mechanical properties to allow easier recycling and utilization on plant. The aim of this study was to gain an understanding of the self-sintering of the BOS filter cake in terms of what reactions occurred, and how strength developed in the filter cake during self-sintering. The approach used was to characterize samples before reaction, and to measure the reactivity of the BOS filter cake during heating in air. Reacted samples were characterized and compared to self-sintered samples from the plant. The BOS filter cake consisted of very fine particles (200–500 nm) of metallic iron and wüstite. Upon heating in air from 100 to 1000 °C, the BOS filter cake underwent a sequence of drying, oxidation, and calcination events. The primary reactions in self-sintering were found to be the oxidation of metallic iron and wüstite to hematite and zinc ferrite, beginning at approximately 120 °C and were largely completed by 500–600 °C. These exothermic oxidation reactions at low temperatures were likely driven by the very fine particle size, and provided the energy required to heat the stockpiles and drive self-sintering. The strength required for recycling the BOS filter cake appeared to result from a network of particle–particle bonds that formed between the very fine iron oxide particles in the matrix during oxidation at elevated temperatures. Temperatures between 600 and 800 °C under oxidizing conditions are likely sufficient to form adequately strong material for transport and recycling in the BOS.








Similar content being viewed by others
References
Nyirenda RL (1991) The processing of steelmaking flue-dust: a review. Miner Eng 4:1003–1025
Hleis D, Fernández-Olmo I, Ledoux F, Kfoury A, Courcot L, Desmonts T, Courcot D (2013) Chemical profile identification of fugitive and confined particle emissions from an integrated iron and steelmaking plant. J Hazard Mater 250–251:246–255
Ahmed HM, Persson A, Ökvist LS, Björkman B (2015) Reduction behaviour of self-reducing blends of in-plant fines in inert atmosphere. ISIJ Int 55:2082–2089
Makkonen HT, Heino J, Laitila L, Hiltunen A, Pöyliö E, Härkki J (2002) Optimisation of steel plant recycling in Finland: dusts, scales and sludge. Resour Conserv Recycl 35:77–84
Nedar L (1996) Dust formation in a BOF converter. Steel Res 67:320–327
Hayes P (1993) Process principles in minerals & materials production. Hayes Publishing, Sherwood, QLD
Suetens T, Guo M, Van Acker K, Blanpain B (2015) Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. J Hazard Mater 287:180–187
Suetens T, Guo M, Van Acker K, Blanpain B (2015) Gas-solid reaction kinetics of ZnFe2O4 formation from 907 to 1100 °C. J Phys Chem A 119:4718–4722
Steer J, Grainger C, Griffiths A, Griffiths M, Heinrich T, Hopkins A (2014) Characterisation of BOS steelmaking dust and techniques for reducing zinc contamination. Ironmaking Steelmaking 41:61–66
Kelebek S, Yörük S, Davis B (2004) Characterisation of basic oxygen furnace dust and zinc removal by acid leaching. Miner Eng 17:285–291
Longbottom RJ, Monaghan BJ, Zhang G, Chew SJ, Pinson DJ (2016) Characterisation of steelplant by-products to realise the value of Fe and Zn. In: Proceedings of the 7th European coke and ironmaking congress, ASMET, Leoben, Austria, pp. 1017–1026
Longbottom RJ, Monaghan BJ, Zhang G, Chew SJ, Pinson DJ (2016) Characterisation and Resource Development of Steel Plant By-Product Streams. Chemeca 2016: Chemical Engineering - Regeneration. Recovery and Reinvention, RACI, Melbourne, pp 352–362
Longbottom RJ, Monaghan BJ, Zhang G, Pinson DJ, Chew SJ (2019) Self-sintering of BOS filter cake for improved recyclability. ISIJ Int 59:432–441
Chun T, Zhu D (2015) New process of pellets-metallized sintering process (PMSP) to treat zinc-bearing dust from iron and steel company. Metall Mater Trans B 46B:1–4
Hay SM, Rankin WJ (1994) Recovery of iron and zinc from blast furnace and basic oxygen furnace dusts: a thermodynamic evaluation. Miner Eng 7:985–1001
Jaafar I, Griffiths AJ, Hopkins AC, Steer JM, Griffiths MH, Sapsford DJ (2011) An evaluation of chlorination for the removal of zinc from steelmaking dusts. Miner Eng 24:1028–1030
Bale CW, Bélisle E, Chartrand P, Decterov S, Eriksson G, Gheribi A, Hack K, Jung I-H, Melancon J, Pelton, AD, Petersen S, Robelin C (2014) Recent developments in FactSage thermochemical software and databases. In: Mackey P, Grimsey E, Jones R, Brooks G (Eds) Celebrating the Megascale: Proceedings of the Extraction and Processing Division Symposium on Pyrometallurgy in Honor of David G.C. Robertson, TMS, Warrendale PA, pp 114–148
Muan A, Osborn EF (1965) Phase equilibria among oxides in steelmaking. Addison-Wesley Publishing, Reading, MA
Ahn YJ, Kim YS, Shin Y (2004) Approximation of circular arcs and offset curves by Bézier curves of high degree. J Comput Appl Math 167:405–416
Wang Z, Pinson D, Chew S, Monaghan BJ, Pownceby MI, Webster NA, Rogers H, Zhang G (2016) Effects of sintering materials and gas conditions on formation of silico-ferrites of calcium and aluminium during iron ore sintering. ISIJ Int 56:1138–1147
Webster NAS, Pownceby MI, Madesn IC, Kimpton JA (2012) Silico-ferrite of calcium and aluminium (SFCA) iron ore sinter bonding phases: new insights into their formation during heating and cooling. Metall Mater Trans B 43B:1344–1357
Acknowledgements
Funding from the Australian Research Council Industrial Transformation Research Hubs Scheme (Project Number IH130100017) is gratefully acknowledged. This research used equipment funded by Australian Research Council grant LE0882813 and located at the UOW Electron Microscopy Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Heat and Mass Balance for Enthalpy calculations
The enthalpies as measured by DSC were compared against the theoretical several reaction schemes, as given in Table 2. The enthalpies were calculated using the measured mass change during the reaction and the initial composition of the BOS filter cake. The overall mass balance used is given in Eq. 2.
where Δm is the measured mass change (from the TGA curve) during the Stage 2, ni is the number of moles of species i that have reacted, as defined in Eqs. 3–5, M(Fe reaction) is the mass change per mole associated with the reaction of iron, M(FeO reaction) is the mass change per mole associated with the reaction of wüstite, and \({\mathrm{M}}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is the mass change per mole associated with the formation of ZnFe2O4. For reaction scheme 1, M(Fe reaction) is associated with reaction 3, M(FeO reaction) is associated with reaction 4, while \({M}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is zero. For reaction scheme 2, M(Fe reaction) is associated with reaction 5, M(FeO reaction) is associated with reaction 5, while \({M}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is associated with reaction 8. For reaction scheme 3, M(Fe reaction) is associated with reaction 9, M(FeO reaction) is zero, while \({M}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is associated with reaction 10.
where fH2O is the mass fraction of water in the unreacted BOS filter cake; and fFe, fFeO, and fZnO are the mass fractions of metallic iron, wüstite, and zinc oxides, respectively, in the BOS filter cake on a dry basis, mreacted is the mass of the BOS filter cake sample that reacted, mo is the original sample mass, and Mi is the molar mass of species i. fFe and fFeO were estimated by semiquantitative XRD to be 0.27 and 0.45, respectively, while fH2O and fZnO values are listed in Table 1. Substituting Eqs. 17–19 into Eq. 16 allowed mreacted to be calculated iteratively, and the values for nFe, nFeO, and \({n}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) to be determined.
These values for nFe, nFeO, and \({n}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) were than used to calculate the enthalpy based on the enthalpies of reaction for each reaction in the different schemes, as given in Eq. 2. In Eq. 2, the values of each ΔH term vary, depending on which reaction scheme was considered. For reaction scheme 1, ΔH(Fe reaction) is associated with reaction 3, ΔH(FeO reaction) is associated with reaction 4, while \({\Delta \mathrm{H}}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is associated with reaction 7. For reaction scheme 2, ΔH(Fe reaction) is associated with reaction 3, ΔH(FeO reaction) is associated with reaction 4, while \({\Delta \mathrm{H}}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is associated with reaction 8. For reaction scheme 3, ΔH(Fe reaction) is associated with reaction 9, ΔH(FeO reaction) is zero, while \({\Delta \mathrm{H}}_{({\mathrm{Z}\mathrm{n}\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4})}\) is associated with reaction 10.
Rights and permissions
About this article
Cite this article
Longbottom, R.J., Monaghan, B.J., Pinson, D.J. et al. Understanding the Self-Sintering Process of BOS Filter Cake for Improving Its Recyclability. J. Sustain. Metall. 5, 429–441 (2019). https://doi.org/10.1007/s40831-019-00233-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00233-x