Abstract
Introduction
Sodium oxybate has been approved by the US Food and Drug Administration (FDA) to treat narcolepsy for 20 years; however, the only available products have been immediate-release (IR) formulations given twice nightly—once at bedtime and a second dose 2.5–4 h later—creating inherent risks with dosing administration errors.
Objectives
Evidence and risks associated with accidental ingestion of the second IR oxybate dose < 2.5 h after the first dose were examined.
Methods
The FDA Adverse Event Reporting System database was searched for “inappropriate schedule of product administration” with IR sodium oxybate or calcium/magnesium/potassium/sodium oxybates; reports classified as serious and with IR oxybate as the suspect product were further analyzed.
Results
Of 541 reports meeting the search criteria, 178 were classified as serious: accidental early administration of the second dose resulting in adverse events (AEs; n = 41); “near miss” (no harm reported following early dosing; n = 9); intentionally taking second dose early (n = 25); other inappropriate use (late dosing/not taking daily; n = 102); and one duplicate report. Of the 41 reports of taking the second dose too early resulting in AEs, 22% (9/41) used emergency services and 27% (11/41) resulted in hospitalizations. AEs reported with accidentally taking the second dose too early included CNS depression, bradycardia, respiratory depression, dizziness, seizure, confusion, delirium, difficulty awakening, drowsiness, falls, nausea, vomiting, and enuresis.
Conclusions
Patients, caregivers, clinicians, and Poison Control Centers should be aware of the risk of accidentally dosing twice-nightly IR oxybates earlier than 2.5 h after the first dose and the subsequent harm that may occur with early dosing.
Plain language summary
Sodium oxybate and calcium, magnesium, potassium, and sodium oxybates (both medicines are called “oxybates”) are medicines used to treat narcolepsy. People with narcolepsy need to take these medicines twice every night. They take one dose at bedtime and then wake up 2.5–4 h later to take the second dose. People could make mistakes taking their oxybate medicine because of this schedule. We looked in the US Food and Drug Administration’s (FDA’s) database called the FDA Adverse Event Reporting System to learn if there were reports of people accidentally taking the second dose of their oxybate medicine earlier than 2.5 h after taking the first dose. People who accidentally took the second dose too early experienced side effects like slowing down of the brain, breathing, and heart; feeling dizzy; having a seizure; being confused; having trouble waking up; being sleepy; falling down; feeling sick to their stomach; throwing up; and wetting the bed. Some people used emergency help and some went to the hospital. We found that harm may occur if people take the second dose of these oxybates too soon after the first dose. These risks were not previously reported. People with narcolepsy, their families, and their doctors need to know about these risks with the oxybates that are taken twice every night.
Similar content being viewed by others
Sodium oxybate has been FDA approved for narcolepsy since 2002 as an immediate-release formulation requiring twice-nightly dosing, with a middle-of-the-night dose taken 2.5–4 h after the first dose per the product label. |
Postmarketing reports show that patients may accidentally take their second dose less than 2.5 h after the first dose, which has been associated with moderate to severe adverse events, sometimes requiring hospitalization or medical treatment in an emergency department. |
This risk is not currently described in immediate-release oxybate product labeling; patients, caregivers, clinicians, and Poison Control Centers should be aware of the risk of accidentally taking the second dose of twice-nightly immediate-release oxybates earlier than 2.5 h after the first dose and the harm that may occur in association with early dosing. |
1 Introduction
Sodium oxybate, the sodium salt of γ-hydroxybutyrate (GHB), has been studied since the 1970s for the treatment of narcolepsy [1]. The US Food and Drug Administration (FDA) approved Xyrem® [sodium oxybate oral solution (Jazz Pharmaceuticals, Inc., Palo Alto, CA, USA)] in 2002 for the treatment of cataplexy (sudden loss of muscle control) in adults with narcolepsy [2]. In 2005, the indication was expanded to treat excessive daytime sleepiness (EDS) [2], and in 2018 use was approved for pediatric patients 7 years and older [3]. In 2020, the FDA approved Xywav® (calcium, magnesium, potassium, and sodium oxybates oral solution (Jazz Pharmaceuticals) or “mixed salt” oxybates) for the same indications [4]. Xyrem and Xywav are only available through a restricted distribution program, the Xywav and Xyrem Risk Evaluation and Mitigation Strategy (REMS), because of the risks of central nervous system (CNS) depression, as well as abuse and misuse. In order for patients to receive Xyrem or Xywav, prescribers must be enrolled and certified in the REMS and patients must be enrolled in the REMS, Xyrem/Xywav can only be dispensed from a REMS-certified pharmacy, and new patients receive extensive screening and counseling prior to first dispense.
GHB is a CNS depressant; the putative mechanism of action for oxybates is mediated through the γ-aminobutyric acid receptor B (GABAB) during sleep at dopaminergic and noradrenergic neurons, as well as at the thalamocortical neurons [5, 6]. Both FDA-approved, commercially available forms are immediate release (IR); because GHB has a short half-life of 30 min–1 h, a two-dose nightly regimen of IR oxybates is required for adequate treatment of narcolepsy symptoms. For both Xyrem and Xywav, patients are instructed to prepare two doses before bedtime, extracting via a dosing syringe the prescribed dose from a 180 mL bottle, which contains 500 mg/mL, and requires dilution with water. Patients take the first dose at bedtime, with most falling asleep 5–10 min later. Per the label, patients are instructed to wake up and take the second dose at least 2.5 h but no more than 4 h after the first bedtime dose. The approved dosage for the treatment of narcolepsy is 6–9 g per night provided in these two divided doses; i.e., 3–4.5 g twice per night.
Narcolepsy is a chronic neurological disease in which the brain cannot regulate sleep-wake cycles [7, 8]. Narcolepsy is a rare disorder, affecting only about 1 in 2000 Americans [9]. Peak symptom onset is often in the teenage years, with diagnosis frequently not occurring until several years later. Although all people with narcolepsy experience EDS, cataplexy is present only in narcolepsy type 1 (NT1). Other classic symptoms for both narcolepsy without cataplexy [narcolepsy type 2 (NT2)] and NT1 may include disrupted night-time sleep, hypnagogic and/or hypnopompic hallucinations (e.g., hallucinations upon falling asleep or waking up, respectively), and sleep paralysis. Patients with narcolepsy also often report “brain fog” and automatic behaviors, which entails carrying out activities and appearing awake, while actually being in a state between wakefulness and sleep [10].
Prescribing information (PI) is a key source of data for healthcare professionals, patients, and caregivers to understand both the benefits and the risks of a medication. Postmarketing experience is updated in product PIs, based on adverse events (AEs) that are reported directly to the manufacturer or received indirectly (e.g., literature reports, FDA notification). The obligation of sponsors is to report AEs to the FDA and identify trends or signals that may necessitate some type of regulatory action, such as updating the PI. In 2014, the Xyrem PI was updated to add the risk of falls, with specific language noting that sudden onset of sleep had “led to falls complicated by injuries, in some cases requiring hospitalization.” The PI was further updated to instruct patients to remain in bed while taking Xyrem and lie down immediately after ingestion of both the first and the second dose of oxybate. In 2022, additional safety updates were added to the PIs for both Xyrem and Xywav regarding the observation of acidosis occurring with higher GHB doses and the use of hemodialysis and other forms of extracorporeal drug removal being performed with GHB doses ≥ 125 g and occurrence of acidosis [5, 6].
Avadel Pharmaceuticals has developed an extended-release formulation of sodium oxybate (LUMRYZ™), which has received FDA tentative approval, and allows for a single bedtime dose. LUMRYZ has been granted Orphan Drug Designation based upon the plausible hypothesis that avoiding middle-of-the-night dosing improves patient safety over IR oxybate formulations. One potential for improved patient safety is avoiding dosing errors related to not waiting 2.5 h after the first IR oxybate dose to take the second dose.
In the pivotal trial of IR mixed-salt oxybates (n = 201), a serious adverse drug reaction was reported after a patient inadvertently took the second dose of oxybate shortly after the first dose; both doses were 4.5 g, totaling the maximum approved total nightly dose of 9 g [11]. The participant experienced confusion and hallucinations and was hospitalized, with discharge after symptom resolution. Similarly, in a postmarketing European publication, an 18-year-old female also had an inappropriate schedule of drug administration/accidental overdose [12]. She took the second dose immediately after the first, resulting in sleepiness, with overnight hospitalization.
Because of the inherent potential for accidental dosing administration errors of this type and resulting patient harm with twice-nightly dosing of IR oxybates, examination of the FDA Adverse Event Reporting System (FAERS) was undertaken to assess the safety risks posed to patients receiving IR oxybates.
2 Methods
2.1 Identification of Relevant Case Reports
FAERS is an electronic database designed to support the FDA’s postmarketing safety surveillance program for all approved drug and therapeutic biologic products. The FAERS database was searched using the FAERS Public Dashboard in the following way: “Search by Product,” using the product names “Xyrem,” “Xywav,” “sodium oxybate,” and “calcium oxybate\magnesium oxybate\potassium oxybate\sodium oxybate.” The list of reported reactions was reviewed to identify the reaction that was mostly likely to capture incidents of taking the second dose not according to the instructions in the PI. The Medical Dictionary for Regulatory Activities [MedDRA®, The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Geneva, Switzerland] preferred term “inappropriate schedule of product administration” was used to identify reports in which the second dose may not have been taken as prescribed. The search criteria were further refined to only include reports in which the oxybate was a “Suspect Product” and for which the case outcome was classified as serious (i.e., death, life-threatening, hospitalization, disability or permanent damage, congenital anomaly/birth defects, or other serious or important medical event that may jeopardize the patient). The FAERS reports were then requested from the FDA under the Freedom of Information Act. All FAERS reports were analyzed by two separate reviewers (author DB and an independent consultant) to adjudicate relevant reports for inclusion, i.e., those in which the patient accidentally took the second dose less than 2.5 h after the first dose and experienced an AE.
3 Results
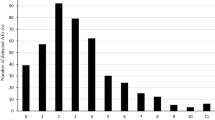
For the FAERS data available through 31 March 2022, there were a total of 541 reports that included the preferred term of “inappropriate schedule of product administration” as a reaction with the IR twice-nightly oxybates. Of the 541 reports identified in FAERS, there were 178 reports in which IR twice-nightly oxybate was the suspect product and the patient had a serious outcome. During the time frame from July 2021 through July 2022, the 178 reports meeting the search criteria were requested and reviewed. Upon review, one FAERS report was identified as a duplicate and was removed from this analysis. Forty-one reports, classified as serious, described patients accidentally taking their second dose of Xyrem less than 2.5 h after the first dose and experiencing AE(s) as a result (Fig. 1). All 41 reports of interest were submitted to the FDA by the manufacturer, Jazz Pharmaceuticals, between August 2011 and November 2020.
A brief description of the 41 reports is included in Table 1. Twenty-six (63%) reports involved female patients; the age of the affected patients ranged from 7 to 65 years, with an average age of 42 years. Nine (22%) reports resulted in emergency medical services or emergency department visits, with all patients discharged, and 11 (27%) reports resulted in hospitalization. Ten (24%) reports were initially reported by the American Association of Poison Control Centers (AAPCC). One report involved a patient who had a tendency to take her second dose 30 min–1.5 h after the first dose; upon her death, Xyrem was noted as possibly contributing.
Timing of dosing errors varied: 20% took their two doses at the same or almost the exact same time; 39% consumed their second Xyrem dose less than 1 h after the first dose; and 61% took the second dose between 1 and 2.5 h after the first dose. Harm to patients was described more frequently when patients took their second dose 1 h or less after their first dose. AEs reported with accidentally taking the second dose too early included CNS depression, bradycardia, respiratory depression, dizziness, seizure, confusion, delirium, difficulty awakening, drowsiness, falls, nausea, vomiting, and enuresis. As noted in the Xyrem PI, a dose-response relationship is stated for nausea, vomiting, paresthesia, disorientation, disturbance in attention, feeling drunk, sleepwalking, and enuresis [5]. Slightly more than half (51%) reported the patient’s event resolved; the outcome was unknown in the remaining reports.
Some narratives described patients being fearful of continuing IR sodium oxybate, with either confirmed discontinuation or consideration of discontinuation, because they feared that the error would occur again. In one report, the patient became fearful after realizing she had taken her dose too soon and forced herself to vomit and remain awake as long as she could to ensure she continued breathing.
Contributing factors to patients consuming the second dose less than 2.5 h after the first included distraction, forgetting, and/or restarted routine; sleepwalking; did not check or readjust alarm clock to make sure enough time passed; misunderstood frequency prescribed or misread directions; and not recognizing that two doses were added to the same container.
For reports not considered relevant for inclusion in this study, nine represented near misses where patients accidentally took the second dose less than 2.5 h after first dose but had no associated AE(s). “Near misses” are events where an error occurs but does not cause harm to the patient because of chance [13]. Additionally, there were 25 reports in which the patient intentionally took the second dose early. The remaining 102 reports involved another inappropriate use of the drug such as taking the second dose more than 4 h after the first dose or not taking the medication daily as prescribed (Fig. 1).
The reports included in this analysis broadly fell into one of three groups: (1) hospitalization, (2) emergency room visit, or (3) no medical attention needed. As an example of the information included in these FAERS system reports, one report from each of these categories is fully described in Table 2.
4 Discussion
The establishment of pharmacovigilance systems, including robust signal management the FDA requires for postmarketing safety surveillance, is critical to monitoring the risk–benefit profile of a drug after receiving FDA approval. Postmarketing reports are valuable to identify hazards with drugs that were not observed or recognized at the time of approval. When a report is both serious and unexpected, meaning the risk is not described in the PI, it meets the FDA’s expedited reporting requirements of 15-day “alert reports” in which a sponsor must report as soon as possible, but no later than 15 calendar days from the initial receipt date of the information. All reports, and particularly expedited (15-day) reports, should be routinely and thoroughly evaluated.
Signal management is a critical part of pharmacovigilance. Signals can be identified for a variety of reasons, including when information identifies a possible causal relationship between an AE and a medication. New, unexpected events may merit updates to product labeling to help ensure safe use of a medication.
In 2009, the manufacturer of Xyrem and Xywav published a safety overview of postmarketing experience. Wang et al. [14] described 26,000 patients globally who received sodium oxybate between 2002 and 2008, noting that illicit GHB has been reported to decrease consciousness and has been associated with overdose, presumably owing to “its relatively steep dose-effect relationship.” Overdose incidents included in that report were primarily related to suicide attempts or off-label use of the drug (e.g., insomnia, fibromyalgia). In 2011, an erratum updating and correcting previously reported safety data was published [15].
The Institute for Safe Medication Practices (ISMP), a nonprofit organization dedicated to the promotion of safe medication practices, published a QuarterWatch® report of signals for certain medications including Xyrem in 2013 [16]. ISMP referenced the Wang publication [14], including the erratum [15], describing the manufacturer’s “optimistic safety overview” of sodium oxybate, noting that in 2011, “Jazz revealed that it had included only 21 of 103 reported patient deaths. The problem was traced to inadequate coordination of reporting procedures with the central pharmacy that distributed the prescriptions” [15]. In the 2011 erratum by Wang et al., overdose (intentional, unintentional, suspected drug overdose with and without sodium oxybate) was the most common reason given among the documented or suspected causes of death. ISMP further described an unexpectedly high number of serious AEs associated with sodium oxybate given the relatively small patient population (approximately 9000 patients), noting that they represented 15% of the exposed population as measured in patient-years. ISMP recognized that close follow-up with the patients could contribute to the high volume. Finally, the ISMP report found that off-label use was documented in at least 19% of the reported AEs and questioned whether the off-label Xyrem use has been or could be controlled.
The FDA’s stated goal of FAERS is “to improve the public health by providing the best available tools for storing and analyzing safety reports” [17]. FAERS reports are evaluated by epidemiologists and other scientists in the Center for Drug Evaluation and Research’s Office of Surveillance and Epidemiology to detect safety signals and to monitor drug safety.
In describing how AEs are monitored at the FDA, the safety evaluators [18], who are specially trained clinical pharmacists, look for signs of safety problems, such as temporal association (i.e., the drug was taken before the AE occurred), coherence with existing information or biological plausibility, similar effect with drugs of the same class, dose–response relationship, consistency of the association (reproducibility of the results), and specificity of the association [18]. When this stated lens is applied to the reports identified in this research, they appear to merit further evaluation (Table 3).
No discussion of inappropriate schedule of product administration (i.e., accidentally taking the second dose less than 2.5 h after the first dose) or related potential patient harm is included in the labeling for the marketed IR twice-nightly oxybate products. The Xyrem and Xywav PIs include boxed warnings regarding CNS depression with respiratory depression and the risks for abuse and misuse [5, 6]. In the section on overdosage in the Xywav PI, the language states that “no cases of overdose (greater than 9 g) were reported” in the pivotal trial, despite the single 9 g dose accidentally taken in that study resulting in hospitalization. The balance of the overdosage sections in the Xyrem and Xywav PIs focus on reports of illicit use of GHB. Of note, the FAERS search yielded cases of interest that were related only to Xyrem; while the same inherent dosing error exists with Xywav, the lack of reports captured may be due to a limited time this medication was available to patients when this research was conducted. Although the PIs do not describe accidentally taking the second dose less than 2.5 h after the first dose, 17% of the 41 reports in this study submitted by the manufacturer were not expedited to the FDA as 15-day alert reports.
There are limitations to this study. Because Xyrem/Xywav requires a REMS, which increases interactions with patients and prescribers, there is an increased likelihood of reporting of AEs. As the FDA notes on the FAERS Public Dashboard, “this surveillance system has limitations, including the potential submission of incomplete, inaccurate, untimely, and/or unverified information”; further, the “incidence or prevalence of an event cannot be determined from this reporting system alone due to potential under-reporting of events and lack of information about frequency of use.”
AE reporting is sometimes referred to as the “tip of the iceberg” because reporting by healthcare professionals, caregivers, and patients is voluntary. Researchers have estimated that the FDA receives reports for only 1–13% of all serious AEs [18]. While the search of FAERS included any reports from the time that Xyrem was FDA approved in 2002, the earliest report of this type was submitted in 2011. A considerably higher number of reports was submitted after the REMS was in place; only six reports were submitted between 2011 and 2014; 35 reports were submitted after 2015. Only reports with the preferred term of “inappropriate schedule of product administration” were retrieved; it is possible that additional search terms (e.g., product administration error, product use issues, overdose) would have yielded additional reports. The FAERS database is updated quarterly; this study represents the current snapshot in time for the dates identified. Only the FAERS database was searched; there may be more reports of a similar nature contained in other sources (e.g., claims databases). While this analysis focused upon accidental dosing errors, consideration should be given that intentional dosing errors may also occur. Lastly, these reports primarily involved patients living with a complicated neurological disorder; no medical records were available for independent documentation of events. Awareness of the potential for accidentally consuming the second oxybate dose less than 2.5 h after the first may help to prevent future errors.
5 Conclusions
Sodium oxybate has been FDA approved for the treatment of narcolepsy since 2002 as an IR formulation and is recognized as standard of care for this debilitating condition [19]. When taken appropriately, sodium oxybate effectively and safely treats the symptoms of narcolepsy. Serious risks can occur with taking the second, middle-of-the-night dose less than 2.5 h after the first dose, which are not described in the labeling for the IR twice-nightly oxybates.
References
Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6(1):1–6. https://doi.org/10.1017/s0317167100119304.
Jazz Pharmaceuticals. Jazz Pharmaceuticals, Inc. Release: Xyrem® Receives FDA Approval for the Treatment of Excessive Daytime Sleepiness in Patients With Narcolepsy. 2005. https://www.biospace.com/article/releases/jazz-pharmaceuticals-inc-release-xyrem-r-receives-fda-approval-for-the-treatment-of-excessive-daytime-sleepiness-in-patients-with-narcolepsy-/. Accessed 15 Jul 2022.
Jazz Pharmaceuticals. Jazz Pharmaceuticals Announces FDA Approval of Xyrem® (sodium oxybate) for the Treatment of Cataplexy or Excessive Daytime Sleepiness in Pediatric Narcolepsy Patients. 2018. https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-fda-approval-xyremr-sodium. Accessed 15 Jul 2022.
Jazz Pharmaceuticals. Jazz Pharmaceuticals Announces U.S. FDA Approval of Xywav™ (calcium, magnesium, potassium, and sodium oxybates) Oral Solution for Cataplexy or Excessive Daytime Sleepiness Associated with Narcolepsy. 2020. http://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-us-fda-approval-xywavtm-calcium. Accessed 15 Oct 2020.
Jazz Pharmaceuticals. XYREM (sodium oxybate oral solution, CIII). Full Prescribing Information. California: Jazz Pharmaceuticals; 2022.
Jazz Pharmaceuticals. XYWAV (calcium, magnesium, potassium, and sodium oxybates). Full Prescribing Information. California: Jazz Pharmaceuticals; 2022.
Kornum BR, Knudsen S, Ollila HM, Pizza F, Jennum PJ, Dauvilliers Y, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3(1):16100. https://doi.org/10.1038/nrdp.2016.100.
Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511. https://doi.org/10.1016/S0140-6736(07)60237-2.
Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. https://doi.org/10.1093/sleep/30.1.13.
Narcolepsy Symptoms: Automatic Behaviors. Health Union. 2020. https://narcolepsy.sleep-disorders.net/symptoms/automatic-behaviors. Accessed 28 Jun 2022.
Bogan RK, Thorpy MJ, Dauvilliers Y, Partinen M, Del Rio Villegas R, Foldvary-Schaefer N, et al. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep. 2021;44(3):zsaa206. https://doi.org/10.1093/sleep/zsaa206.
Mayer G, Plazzi G, Iranzo A, Ortega-Albas J, Quinnell T, Pesch H, et al. Long-term compliance, safety, and tolerability of sodium oxybate treatment in patients with narcolepsy type 1: a postauthorization, noninterventional surveillance study. Sleep. 2018;(9). https://doi.org/10.1093/sleep/zsy128.
Crane S, Sloane PD, Elder NC, Cohen LW, Laughtenschlager N, Zimmerman S. Implementing Near-Miss Reporting and Improvement Tracking in Primary Care Practices: Lessons Learned. Agency for Healthcare Research and Quality, Rockville, MD. 2017. https://www.ahrq.gov/patient-safety/resources/liability/crane.html. Accessed 4 Aug 2022.
Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med. 2009;5(4):365–71.
Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Sodium oxybate: updates and correction to previously published safety data. J Clin Sleep Med. 2011;7(4):415–6. https://doi.org/10.5664/JCSM.1214.
Institute for Safe Medication Practices. Quarterwatch™ Report (Quarters 2 and 3, 2013): Signals for Chantix, Xyrem, Gilenya, and Tecfidera. 2014. https://www.ismp.org/resources/quarterwatchtm-report-quarters-2-and-3-2013-signals-chantix-xyrem-gilenya-and-tecfidera. Accessed 28 Jun 2022.
US Food and Drug Administration. Postmarketing Surveillance Programs. 2020. https://www.fda.gov/drugs/surveillance/postmarketing-surveillance-programs. Accessed 28 Jun 2022.
Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18(1):57–60. https://doi.org/10.1046/j.1525-1497.2003.20130.x.
Maski K, Trotti LM, Kotagal S, Robert Auger R, Rowley JA, Hashmi SD, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(9):1881–93. https://doi.org/10.5664/jcsm.9328.
Acknowledgements
Editorial support was provided by The Curry Rockefeller Group, LLC (Tarrytown, NY), and was funded by Avadel Pharmaceuticals (Chesterfield, MO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Avadel Pharmaceuticals (Chesterfield, MO) funded editorial support provided by The Curry Rockefeller Group, LLC (Tarrytown, NY) and the open access fee for this research.
Conflicts of interests/competing interest
The authors are employees of Avadel Pharmaceuticals.
Availability of data and material
The datasets analyzed during the current study are available in the FDA Adverse Event Reporting System (FAERS) database, available at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard, and will be shared on reasonable request to the corresponding author.
Ethics approval
This research study was conducted retrospectively from publicly available data and ethics approval was not needed.
Consent to publish/participate
Not applicable.
Code availability
Not applicable.
Author contributions
The authors confirm responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and writing the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gudeman, J., Burroughs, D. Evidence of Accidental Dosing Errors with Immediate-Release Sodium Oxybate: Data from the US Food and Drug Administration Adverse Event Reporting System. Drugs - Real World Outcomes 10, 225–234 (2023). https://doi.org/10.1007/s40801-023-00351-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00351-9