Abstract
Background
Abemaciclib is the most recent oral cyclin-dependent kinase 4 and 6 inhibitor (CDK4 & 6i) to receive US Food and Drug Administration (FDA) approval to treat hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced or metastatic breast cancer (MBC). Administrative claims data were used to describe patient characteristics and select clinical and economic outcomes in US patients treated in routine clinical practice. Prior analyses from electronic health records data indicate approximately 25% of patients received either palbociclib or ribociclib for MBC before initiating abemaciclib treatment; this work further explored these findings and associated outcomes.
Methods
This retrospective study analyzed medical and pharmacy claims from the IBM® MarketScan® Research Databases between 1 January 2007 to 31 January 2020. Patients with HR+, HER2− MBC newly initiating abemaciclib between 1 September 2017 and 31 October 2019 were included and grouped by concomitant therapy (+aromatase inhibitor (AI), +fulvestrant (F), 200 mg abemaciclib monotherapy (Mono), or +other), and outcomes were analyzed by prior CDK4 & 6i use. Patient and treatment characteristics were summarized with descriptive statistics. Kaplan–Meier methods assessed time-to-discontinuation (TTD; i.e., persistency) and time-to-chemotherapy (TTC). Adherence (defined by the medication possession ratio) and drug wastage were determined.
Results
This analysis included 454 patients (mean age 57.7 years), with 35.0% (n = 159) in the +F group, 29.3% (n = 133) in the +AI group, 10.4% (n = 47) in the 200 mg Mono group, and 25.3% (n = 115) in the +other group. Prior chemotherapy and CDK 4 & 6i use were present in 23.8% and 49.8% of all patients, respectively. Visceral metastases were present at abemaciclib initiation in 50.4% in the +AI group; 49.7% in the +F group; and 55.3% in the 200 mg Mono group. Liver metastases were present in 33.7% of the overall population. Among patients without prior CDK4 & 6i use, the median TTD for patients receiving abemaciclib + AI was not reached [95% CI 430–not reached (NR) days], abemaciclib + F [531 days (95% CI 281–NR)], and abemaciclib mono [141 days (95% CI 80–NR)]. Median TTC for abemaciclib + AI and abemaciclib + F groups were not reached and the median TTC for abemaciclib mono was 535 days (95% CI 181–NR). Medication adherence was 88.7% and medication wastage costs among those with at least one dose modification were $808.12 and $452.2 per patient per month based on amount paid and wholesale acquisition cost (WAC), respectively. Mean length of follow-up for all patients was 350 days (SD 187).
Conclusion
These real-world data complement clinical trial results by examining abemaciclib use among patients treated in routine clinical practice. The sizeable number of patients treated with prior CDK4 & 6i, chemotherapy, and/or visceral metastases at abemaciclib initiation suggest that many patients had very advanced disease and/or were in later stages of their treatment. These data confirm a higher percentage of patients treated with previous CDK4 & 6i than reported previously, reinforcing the importance of the ongoing, prospective clinical trials evaluating outcomes following progression on CDK4 & 6i.
Similar content being viewed by others
These data confirm a higher percentage of patients treated with previous CDK4 & 6i than reported previously, reinforcing the importance of the ongoing, prospective clinical trials evaluating outcomes following progression on CDK4 & 6i. |
The sizeable number of patients treated with prior CDK4 & 6i, chemotherapy, and/or visceral metastases at abemaciclib initiation suggest that many patients had very advanced disease and/or were in later stages of their treatment. |
Our study suggests that patients treated with abemaciclib for MBC were adherent and/or persistent on therapy. |
1 Introduction
Goals for metastatic breast cancer (MBC) treatment include prolonging survival while maintaining quality of life, persistence on therapy, and delaying initiation of chemotherapy when possible [1]. The management of MBC has evolved in recent years with the US regulatory approval and availability of CDK4 & 6 inhibitors (CDK4 & 6i); abemaciclib, palbociclib, and ribociclib are indicated for the treatment of HR+, HER2− locally advanced or MBC in combination with an aromatase inhibitor or fulvestrant based on results of MONARCH [2, 3], PALOMA [4, 5], and MONALEESA [6, 7] clinical trials, respectively. In addition, abemaciclib is approved as monotherapy for the treatment of HR+, HER2− advanced or MBC with disease progression following endocrine therapy (ET) and prior chemotherapy. The recommended MBC starting dose of abemaciclib in combination with an aromatase inhibitor or fulvestrant is 150 mg twice daily and 200 mg twice daily as monotherapy [8]. Abemaciclib is also approved in combination with ET for the adjuvant treatment of adult patients with HR+, HER2−, node-positive early breast cancer (EBC) at high risk of recurrence and a Ki-67 score ≥ 20% as determined by a US Food and Drug Administration (FDA)-approved test [8].
Treatment guidelines support the use of an aromatase inhibitor or fulvestrant in combination with a CDK4 & 6i for first-line HR+, HER2− MBC, and fulvestrant in combination with a CDK4 & 6i in second and subsequent lines if a CDK4 & 6i was not utilized in a prior line of therapy. Guidelines recommend continuing treatment until intolerable toxicity or disease progression. There are limited data to support treatment with a CDK4 & 6i following progression on a CDK4 & 6i, and clinical trials are underway to clarify optimal strategies [9,10,11,12,13,14]; neither palbociclib, ribociclib, nor abemaciclib are approved by the FDA for use in this way.
Real-world data can be a useful complement to randomized studies to characterizing treatment utilization and outcomes of patients treated in routine practice. Real-world studies assessing patterns of use and effectiveness outcomes with abemaciclib have recently become available, including studies evaluating outcomes related to abemaciclib following palbociclib in limited numbers of patients with MBC [15,16,17,18,19,20]. Prior analyses of electronic medical record data in 118 patients with HR+, HER2− MBC treated with abemaciclib indicate that approximately 25% of patients receive either palbociclib or ribociclib as prior treatment for MBC; however, these data are from the first 11 months following initial FDA approval [21]. Additional analyses with larger numbers of patients and longer follow-up are important to understanding whether these patterns of treatment continue as well as associated outcomes. The purpose of this study was to build on this information by characterizing the percentage of US patients with HR+, HER2− MBC treated with abemaciclib who received prior treatment with either palbociclib or ribociclib for MBC, and report their associated persistency, adherence, and time to chemotherapy (TTC) using an administrative claims dataset in the first 2 years following FDA approval.
2 Methods
2.1 Study Design and Data Source
Retrospective analyses were conducted using de-identified, medical and pharmacy claims from the IBM® MarketScan® Research Databases (Commercial and Medicare Supplemental), Early View dataset. Patients with MBC initiating therapy with abemaciclib from 1 September 2017 through 31 October 2019 were identified. The date of a patient’s first adjudicated abemaciclib claim was characterized as the Index Date. Patient characteristics, treatment attributes, as well as select clinical and economic outcomes were measured from (and including) the index date until the end of enrollment (31 January 2020), permitting 30-day gaps during the follow-up period, as illustrated in Fig. 1.
2.2 Study Population and Eligibility Criteria
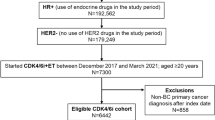
Female or male patients with MBC newly initiating abemaciclib were included. Patients were required to be age ≥ 18 years as of the index date and have at least two claims for a diagnosis of breast cancer at least 30 days apart during the study period, in addition to at least two medical claims for a secondary neoplasm on at least two separate dates at any time during the study period. Key criteria also included HR+ status as evidenced by at least one claim for endocrine therapy or HR+ related diagnosis codes, HER2− status as evidenced by the absence of therapies suggesting HER2 positivity, and continuous enrolment (permitting 30-day gaps) in medical and pharmacy benefits for at least 6 months before and at least 90 days after the index date. For a full list of primary breast cancer and secondary neoplasm diagnosis codes used in this study see Online Supplementary Material (OSM) Table 1. Patients were excluded if there was evidence of claims for two or more strengths of abemaciclib on index date, claims for either palbociclib or ribociclib on index date, or negative payment for abemaciclib (OSM Fig. 1).
2.3 Abemaciclib Treatment Regimen
Each patient was categorized to one of the following abemaciclib regimen cohorts based on their claims history: abemaciclib + AI (AI; letrozole, anastrozole, or exemestane), abemaciclib + fulvestrant (F), abemaciclib 200 mg monotherapy (mono), or abemaciclib + other. During protocol development, regimen rules were determined based on expertise of the study team related to claims adjudication and review of days’ supply information from pharmacy claims for AIs. Cut-off days for categorizing each combination with abemaciclib were derived by conducting feasibility analyses on patient-level data prior to evaluating outcomes.
2.3.1 Abemaciclib + AI
Patients categorized into the abemaciclib + AI regimen cohort were defined as having ≥ 1 prescription fill of an AI from as early as 45 days prior to index date and no later than 45 days afterward, and a subsequent fill of the same AI occurring within the first 120 days following index date (including index date). Patients were not included in this regimen cohort if they received a prescription for 200 mg of abemaciclib on index date or fulvestrant within the first 28 days of index date.
2.3.2 Abemaciclib + Fulvestrant
Patients categorized into the abemaciclib + F regimen were defined as having a claim for fulvestrant on index date or within 27 days following index date. Patients were not included in this regimen cohort if they had a prescription for 200 mg of abemaciclib on index date or met the definition of +AI cohort as mentioned above.
2.3.3 Abemaciclib Monotherapy
Patients categorized into the abemaciclib mono regimen were defined as having 200 mg strength on index date. Patients were not included in this regimen cohort if they met the definitions of either the +AI or +F cohorts.
2.3.4 Abemaciclib + Other
Patients categorized into the abemaciclib + other regimen were defined as having an abemaciclib claim on index date and not meeting the criteria for any of the regimen groups previously described.
2.4 Patient and Prior Treatment Characteristics
Demographic variables described at index included age, sex, geographic location, and insurance type. The Charlson Comorbidity Index (CCI) score [22] and the number and percentage of patients with each CCI comorbidity were determined from all data available prior to the index date [23]. Additional clinical characteristics of interest assessed included history of diagnosis of osteoporosis, Alzheimer’s disease or dementia, depression, anxiety, and history of primary tumors other than breast cancer. Sites of metastasis (i.e., bone-only, brain, liver, lung, and visceral) during the 6-month pre-index period were also described and were defined by evidence of ≥ one diagnosis code. Codes used to define clinical characteristics are provided in OSM Table 2.
For female patients, postmenopausal status (either natural or via medical or surgical castration) was defined as meeting at least one of the following criteria: (1) age ≥ 60 years on index date, (2) evidence of bilateral oophorectomy any time prior to index date, (3) a diagnosis/procedure code related to postmenopausal status any time prior index date, (4) evidence of gonadotropin-releasing hormone (GnRH) analog use 15 days before index date through follow-up period. Premenopausal patients were defined as patients without an indicator of postmenopausal status.
Use of anticancer agents prior to abemaciclib initiation in either the EBC or MBC setting was reported. Codes used to define anticancer agents are provided in OSM Table 3.
2.5 Persistent Treatment Period
The persistent treatment period (PTP) or “at-risk time” was defined as the time between the index date (including index date) until the earlier of date of discontinuation or end of enrollment (permitting 30-day gaps) for censored patients. Data for frequency of dose modification, adherence, and wastage were analyzed based on the amount of time at risk for CDK4 & 6i discontinuation.
2.6 Treatment Persistence, Adherence, and Second Fill Rate
Time to treatment discontinuation (i.e., persistence) was measured from the time from index date to the date of discontinuation. Patients were defined as having discontinued abemaciclib if a different CDK4 & 6i was introduced or a 60‐day gap was observed after exhaustion of the days' supply from the last prescription dispensed. Patients without a discontinuation event were censored at their last day of medication supply. Stockpiling rules were applied that assumed that the patient was “stockpiling” (i.e., using all drugs on hand) if there was an early fill with the same abemaciclib strength [National Drug Code (NDC)]; if a different strength was introduced, it was assumed that the patient discarded remaining medication from previous script fill(s) and the days’ supply was accordingly adjusted in the dataset.
Drug refills in prior analyses of administrative records have been associated with adherence [24]. Therefore, for the purposes of this analysis, adherence was evaluated using the medication possession ratio (MPR) [25] among patients who had at least two or more abemaciclib fills. MPR was calculated as the sum of days of supply of abemaciclib (i.e., the number of treated days) divided by the total number of days from (including) the index date to the earlier of treatment discontinuation or disenrollment.
Evidence of a second claim for abemaciclib within 90 days following index date (limited to patients with days’ supply ≤ 30 days on index date) was used to calculate the second fill rate.
2.7 Treatment Strength and Dose Modification
The medication strength associated with the abemaciclib claim on index date was used as the index dose. Dose modification (an increase or decrease in dose) was defined as a change in daily dose of ≥ 50 mg abemaciclib compared to the previous dose. The magnitude of the dose modification was calculated as the previous daily dose minus the new daily dose. Dose modification was calculated within the first 90 days, among patients with at least two claims during the study period. Time to first dose modification was determined among those with a dose modification.
2.8 Time to Chemotherapy
Time to chemotherapy was defined as the time from index date to the initial claim for chemotherapy, for patients whose abemaciclib regimen did not contain chemotherapy. Patients were censored at end of enrollment if no claim for chemotherapy was recorded. For chemotherapy medications and their codes used in this study see OSM Table 4.
2.9 Wastage Analysis
Wastage was evaluated for patients with at least one dose modification of abemaciclib. Instances of wastage were identified by the presence of > one abemaciclib prescription fill with different NDC codes. For two adjacent script fills with different NDC codes, the number of overlapping days between the days’ supply of the first fill and the start of the next fill was determined as previously described [26]. The number of overlapping days’ supply was multiplied by the wholesale acquisition cost (WAC) [27] per tablet of the earlier of the two fills × 2 (to account for two doses per day). Wastage per patient per month (PPPM) was calculated as the sum of wastage costs divided by the sum of the PTP for CDK4 & 6i discontinuation for patients who had different overlapping doses/NDCs. The Consumer Price Index (CPI) Medical Care Component was used to adjusts costs for inflation over time, using 2019 US dollars ($).
2.10 Statistical Analyses
Descriptive statistics including means, standard deviations, medians, interquartile ranges, and minimum and maximum values (as applicable) for continuous variables (e.g., age) as well as frequencies and percentages for categorical variables (e.g., region) were reported. Patient characteristics were summarized by abemaciclib regimen and prior CDK4 & 6i use (yes/no) for the overall population. Due to the heterogeneity of treatment regimens that comprise the + other group and subsequent challenges with interpretation of results, these data were not reported for clinical and economic outcomes reported herein. The Kaplan–Meier product limit estimator was used to characterize time-to-event outcomes unless specified otherwise. Analyses were performed using the Instant Health Data (IHD) software (Panalgo, Boston, MA, USA) and R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Study Cohort
Patients who met inclusion/exclusion criteria (Fig. 2) were categorized into one of four abemaciclib treatment regimens on index date; 159 (35%) received abemaciclib + F treatment, 133 (29.3%) received abemaciclib + AI treatment, 115 (25.3%) received abemaciclib + other treatment, and 47 (10.4%) received abemaciclib mono (Fig. 2). Overall, prior use of any other CDK4 & 6i at index date was noted in 226/454 patients (49.8%), including 79 (49.7%) patients within the abemaciclib + fulvestrant cohort as well as 50 (37.6%), 28 (59.6%), and 69 (60.0%) patients in the abemaciclib + AI, abemaciclib mono, abemaciclib + other cohorts, respectively. Drug combinations comprising the other regimen subgroup are noted in OSM Fig. 2.
3.2 Patient Demographics
The study population was predominantly female (n = 449, 98.9%) and the mean (SD) age was 57.7 (10.8) years. Most patients included in this study resided in the northeast (23.4%) and southern regions (31.9%) of the USA. Mean length of follow-up for all patients was 350 days (SD 187). Patient baseline characteristics stratified by abemaciclib regimen and prior CDK4 & 6i use for the overall population are presented in Table 1.
3.3 Clinical Characteristics
Of the females in the overall study population, the majority were post-menopausal (64.6%). The most common sites for metastases that were evaluated during the 6-month pre-index period were bone (76.2%), visceral (50.0%), liver (33.7%), bone only (22.5%), lung (18.5%), and brain (11.5%). Liver metastasis was present in 33.7% (n = 153) of the population overall with the highest frequency observed in patients in the abemaciclib mono group (40.4%), followed by +F (34.0%) and +AI (28.6%). Other anticancer agents used in the 6 months prior to abemaciclib initiation included aromatase inhibitors (59.0%), fulvestrant (32.2%), tamoxifen (10.1%), everolimus (6.6%), and olaparib (1.1%). Prior chemotherapy use was present in 23.8% of the population overall with the highest use in patients treated with abemaciclib mono (51.1%), followed by 18.8% and 21.4% in the +AI, and +F regimens, respectively. The mean (SD) CCI across cohorts was 1.9 (1.9); of comorbidities assessed, the most frequent Charlson comorbidities were mild liver disease (24.2%) and chronic pulmonary disease (12.6%), and the most frequent non-Charlson comorbidity was anxiety (20.9%) (data not shown). Patient clinical characteristics stratified by abemaciclib regimen and prior CDK4 & 6i use for the overall population are presented in Table 2.
3.4 Treatment Attributes
Of the 454 patients, 72.9% (n = 331) initiated abemaciclib at an index dose of 150 mg. Of abemaciclib + AI (n = 133) and abemaciclib + fulvestrant (n = 159) users, 85.0% and 86.8% initiated abemaciclib at an index dose of 150 mg, respectively. For a summary of index doses for abemaciclib regimens stratified by prior CDK4 & 6i use, see Table 3. For patients who received either abemaciclib +AI or +F, a higher percentage of patients who did not receive a prior CDK 4 & 6i received abemaciclib at the recommended starting dose versus patients who received a prior CDK4 & 6i (90.4% vs. 76.0% abemaciclib + AI; 91.3% vs. 82.3% abemaciclib + F).
In the first 90 days post-index, 30.8% (n = 120) of patients in the overall study population had at least one dose modification; 1.8% (n = 7) had at least one dose increase, 29.2% (n = 114) had at least one dose reduction. During the PTP, 38.5% (n = 150) of patients had at least one dose modification; 4.6% (n = 18) had ≥ one dose increase and 36.2% (n = 141) experienced ≥ one dose reduction. The median magnitude of initial dose reduction was 50 mg (interquartile range (IQR) 50–50). The median time to first dose reduction was 50 days (IQR 30–81 days).
3.5 Second Fill Rate and Medication Adherence
Of the 454 patients examined in this study, 85.8% (n = 386) refilled their prescription at least once and 54.4% (n = 247) of patients discontinued treatment with abemaciclib during the follow-up period. The most common indicator of abemaciclib discontinuation was a 60-day gap in therapy [87.9% (n = 217)] versus treatment initiation of a different CDK4 & 6i [12.1% (n = 30)]. The proportion of the overall population who had at least two prescription fills and had an MPR of ≥ 80% was 88.1% (prior CDK4 & 6i, 86.5%; no prior CDK4 & 6i, 88.7%) (Table 4).
3.6 Time-to-Event Outcomes
For patients with no prior CDK4 & 6i use, the median TTD for patients receiving abemaciclib + AI was not reached (95% CI 430–NR days). Median TTDs for remaining treatment regimens were as follows: abemaciclib + F [531 days (95% CI 281–NR)] and abemaciclib mono [141 days (95% CI 80–NR)]. Median time to subsequent chemotherapy (TTC) for abemaciclib + AI and abemaciclib + F groups were not reached and the median TTC for abemaciclib mono was 535 days (95% CI 181–NR). TTD among abemaciclib initiators by regimen group stratified according to prior versus no prior use of CDK4 & 6i is illustrated in Fig. 3. A summary of the TTD and TTC results stratified by CDK 4 & 6i use is presented in Table 5.
Kaplan–Meier curves of time-to-treatment discontinuation (TTD) among abemaciclib initiators by regimen group and stratified according CDK4 & 6i use.  indicates no prior use of any CDK4 & 6i;
indicates no prior use of any CDK4 & 6i;  indicates prior use of a CDK4 & 6i. CDK4 & 6i cyclin-dependent kinase 4 & 6 inhibitor, AI aromatase inhibitor, F fulvestrant, Mono monotherapy, NR not reached
indicates prior use of a CDK4 & 6i. CDK4 & 6i cyclin-dependent kinase 4 & 6 inhibitor, AI aromatase inhibitor, F fulvestrant, Mono monotherapy, NR not reached
3.7 Drug Wastage
Of the 386 patients with a refill, 88 (22.8%) had overlapping days of supply between different abemaciclib doses, generating a median medication wastage cost per patient (with at least one dose modification) of $5059.61 based on the total amount paid by payers and $2541.70 based on WAC. Among patients with at least one dose modification, total PPPM wastage costs by amount paid and WAC were $808.12, and $452.15, respectively (OSM Table 5).
4 Discussion
These data add to the body of evidence for patients with HR+, HER2− MBC treated in routine clinical practice with abemaciclib in the 24 months following initial FDA approval. Patients tended to have characteristics indicative of a worse prognosis, including but not limited to significant prior chemotherapy, CDK4 & 6i use, and visceral metastases. Approximately 50% of patients in this dataset received either palbociclib or ribociclib for MBC prior to treatment with abemaciclib, which to our knowledge is the highest observed in any dataset to date. There are few published data on outcomes related to the continued use of a CDK4 & 6i after progression on a different CDK4 & 6i; however, prospective studies are ongoing to help answer this important clinical question [15, 16, 18, 19]. Characterizing prior CDK4 & 6i use in this study was very important to interpreting results, given the sizable number of patients treated with this approach and the likely situation that they were further out in their disease course when they received abemaciclib. We were challenged in this dataset to characterize abemaciclib use by line of therapy given methodologic limitations (i.e., identifying the patient’s initial metastatic diagnosis date to anchor the start of first line [28, 29]); however, describing the extent of prior therapy, non-cancer comorbidities, and sites of metastases helps characterize the disease status of patients at abemaciclib initiation. While adherence and frequency of metastases between groups was similar, patients who received prior CDK4 & 6i had a higher non-cancer comorbidity burden as noted by the CCI score and a higher frequency of prior chemotherapy use; TTD and TTC results should be interpreted in this context. Despite these factors, our results demonstrate adherence and persistence associated with abemaciclib treatment, and a delay in subsequent chemotherapy initiation in patients with very advanced breast cancer.
Evidence generated from this study is consistent and complementary to EMR-based analyses of abemaciclib use in the period following initial FDA approval for MBC. In an analysis of data from Flatiron Health from September 2017 to December 2018 [21], approximately 50% of patients had visceral metastases at abemaciclib initiation. Among patients who received abemaciclib in ≥ 2 L for MBC, 35% and 50% received a prior CDK4 & 6i and prior chemotherapy, respectively, while 16% received both prior CDK4 & 6i and prior chemotherapy in the metastatic setting [21]. During a similar timeframe, data were analyzed from patients treated with abemaciclib, palbociclib, or ribociclib within the US Oncology Network. While there was a greater frequency of patients receiving abemaciclib in earlier lines of treatment for MBC, a statistically significantly greater percentage of patients (54%) had visceral metastases at initiation compared with palbociclib (39%) or ribociclib (42%) and fewer bone-only metastases (p < 0.05) [30]. These observed patterns for abemaciclib are consistent with observations that new drugs are often offered to sicker patients immediately following FDA approval, including those with more severe disease and a poorer prognosis [31]. Relevant factors also include the fact that abemaciclib is the most recent CDK4 & 6i approved, increasing the possibility that patients received prior treatment with palbociclib or ribociclib and were therefore later in their disease course when they received abemaciclib treatment. Additionally, abemaciclib is the only approved CDK4 & 6i as monotherapy in patients following chemotherapy. Details of the population are important to the interpretation of outcomes, and it will be important to evaluate whether characteristics of patients receiving abemaciclib for MBC evolve over time.
Approximately 85% of patients in the abemaciclib + AI and abemaciclib + F cohorts had a starting dose of 150 mg of abemaciclib, consistent with prescribing information [8]. For patients who received prior CDK4 & 6i, we observed a lower frequency of those starting on the recommended initial dose for abemaciclib, which may be attributable to the advanced disease status of those patients, poor performance status, and/or a reduced threshold for side effects because of these factors. Overall, 30.8% of patients required dose modifications in the first 90 days of therapy with the majority experiencing a dose reduction (29.2%), which is relatively consistent with studies of palbociclib and ribociclib in patients with MBC [13, 26, 32]. The frequency of dose reduction observed for abemaciclib was lower than in the abemaciclib arms from MONARCH 2 (42.9%) and MONARCH 3 (43.4%) clinical trials [33], likely due to a lack of strict protocols guiding dose reduction in the real-world setting as well as potentially the reduced observation period of 90 days for this particular analysis. Additionally, while there is no published real-world evidence characterizing the effectiveness of abemaciclib in patients requiring dose reductions, analyses from MONARCH 2 and MONARCH 3 suggest no negative impact on progression-free survival for patients requiring dose reduction [33].
Drug wastage may be experienced by patients due to a variety of factors including dose modifications and noncompliance [34]. Dose modifications are often recommended and utilized to manage toxicities and establish a tolerable dose for patients taking oral oncolytics. Modifications can occur multiple times during a course of treatment, resulting in unwarranted drug wastage, particularly with medications that cannot be split or reused [26]. In this study, a median PTP of 6.5 months (IQR 4.1–11.5) for CDK4 & 6i discontinuation was observed, resulting in a median total wastage per patient of $5059.61 for the 22.8% who had overlapping days of supply of different abemaciclib doses. While comparative data for CDK4 & 6i are not available, retrospective data previously generated for palbociclib demonstrate wastage costs in the range of $2592–$5471 [26, 35, 36]. As providers and patients become more proficient in managing these treatments over time, it will be important to monitor trends related to drug wastage given cost implications to the healthcare system and to patients.
Medication adherence and persistence are essential to achieving a clinical benefit, and irregularities have been associated with increased healthcare costs and adverse outcomes [34]. One-third of patients with MBC have reported medication nonadherent behaviour [37]. In this study, approximately 85% of abemaciclib initiators (regardless of prior CDK4 & 6i use or regimen) refilled their prescription within 90 days. Furthermore, the proportion of patients achieving an MPR ≥ 80% ranged from 77 to 92% across regimens regardless of prior CDK4 & 6i use. These data are consistent with analyses of MPR related to abemaciclib from a large specialty pharmacy from 1 July 2018 to 31 December 2019 where there was no significant difference (p = 0.858) in mean MPR (0.91) between the intervention and standard-of-care groups [38]. As drug refills in administrative records are associated with adherence [24], and an MPR ≥ 80% is defined as the threshold cut-off for determining adherence [39], these data suggest good patient adherence to abemaciclib.
TTD has been regarded as a surrogate endpoint of effectiveness and may also reflect the safety and tolerability of the drug in the real world [40, 41]. Our analyses revealed that the median TTD for patients receiving abemaciclib + AI with no prior CDK4 & 6i use was not yet reached (95% CI 430–NR days). This study demonstrated a median TTD for patients receiving abemaciclib + F of 531 days (95% CI 281–NR days). Additional follow-up is warranted to confirm whether these trajectories continue over time. As anticipated, median TTD for abemaciclib in patients who received prior CDK4 & 6i was lower than for patients who were newly treated with abemaciclib as these patients likely had a heavier burden of disease. Evaluation of TTD by line of therapy in these populations would be valuable to consider for future analyses of electronic medical records as this information cannot be reliably characterized in claims data and is an important caveat to interpretation. It is also important, as noted above, to await results of ongoing prospective studies so that the most appropriate approach to sequencing treatments is fully elucidated, especially given the FDA approval of abemaciclib in the EBC setting.
Delaying the initiation of chemotherapy in patients with MBC may translate to improvements in quality of life and a reduction in side effects, while lowering emergency department visits and hospitalizations resulting in healthcare cost savings [42, 43]. The ability to delay chemotherapy is considered an important treatment goal for patients, clinicians, and policy makers [44]. Data from MONARCH 2 and MONARCH 3 trials demonstrated a delay in chemotherapy for abemaciclib in combination with fulvestrant and an aromatase inhibitor [45, 46]. In this study, a median TTC for abemaciclib + AI and abemaciclib + F with no prior use of CDK4 & 6i was not reached. These findings complement pivotal abemaciclib controlled trials and may suggest that these regimens delay the need for chemotherapy in a real-world setting; however, longer follow-up time is needed to confirm this observation.
5 Limitations
These results are subject to limitations inherent in analyzing administrative claims data including the lack of clinical indicators of disease severity, such as documentation of disease progression, reasons for discontinuation (toxicity or progression) of CDK4 & 6i, performance status, and line of therapy. Additionally, retrospective claims data are susceptible to coding errors and differences in billing practices, and our analysis is based on coding practices of the provider. As a result, there is limited sensitivity for the use of secondary malignant neoplasm (metastasis) codes, which can cause potential misclassification and may lead to biased assessment of patient characteristics (i.e., sites of metastasis). While the outcome analyses based on prior versus no prior use of CDK4 & 6i were intended to be descriptive and observe trends, they did not control for demographic or clinical differences that may influence the observed results. Lastly, the IBM® MarketScan® Database comprises members in commercial insurance plans; generalizability to other populations may be limited. Larger subgroup samples and additional follow-up time will be valuable to observe whether trends presented herein persist over time. Data from ongoing prospective and retrospective studies in breast cancer may also have an impact on real-world treatment patterns and reinforce the importance of repeating such analyses in the future.
6 Conclusion
These data add to the body of evidence for abemaciclib in MBC by demonstrating that in the 24 months post FDA approval, patients tended to have a relatively high visceral disease burden as well as extensive prior treatment with palbociclib, ribociclib, and/or chemotherapy. These are some of the first data generated from administrative claims for abemaciclib with regard to TTD, TTC, second fill, and adherence based on prior CDK4 & 6i use. Line of therapy is not well characterized in these datasets; given the importance to interpretation, this merits future study where this information can be more robustly characterized. Dosing regimens were mostly consistent with prescribing information across abemaciclib regimens. These data suggest medication adherence and persistence on abemaciclib treatment, while potentially delaying the need for chemotherapy in a real-world setting. Overall, these real-world data complement pivotal abemaciclib clinical trial results by examining abemaciclib use in patients treated in routine clinical practice.
References
Brufsky AM. Delaying chemotherapy in the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Med Insights Oncol. 2015;9:137–47. https://doi.org/10.4137/cmo.S31586.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–84. https://doi.org/10.1200/jco.2017.73.7585.
Johnston S, Martin M, Di Leo A, Im S-A, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. https://doi.org/10.1038/s41523-018-0097-z.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. https://doi.org/10.1016/s1470-2045(15)00613-0.
Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36. https://doi.org/10.1056/NEJMoa1607303.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72. https://doi.org/10.1200/JCO.2018.78.9909.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–7. https://doi.org/10.1093/annonc/mdy155.
Eli Lilly and Company. Verzenio™ (Abemaciclib)[package insert]. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf. Accessed 29 Apr 2022.
ClinicalTrials.gov. Palbociclib after CDK and endocrine therapy (PACE). https://clinicaltrials.gov/ct2/show/NCT03147287. Accessed 28 Apr 2022.
ClinicalTrials.gov. Study of efficacy of ribociclib after progression on CDK4/6 inhibition in patients with HR+ HER2− advanced breast cancer (MAINTAIN). https://clinicaltrials.gov/ct2/show/NCT02632045. Accessed 28 Apr 2022.
NCCN guidelines for patients: Breast Cancer Metastatic. 2022. https://NCCN.org/patients. Accessed 28 Apr 2022.
Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018;20(1):37. https://doi.org/10.1186/s13058-018-0958-2.
Varella L, Eziokwu AS, Jia X, Kruse M, Moore HCF, Budd GT, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176(2):429–34. https://doi.org/10.1007/s10549-019-05176-1.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidlines): Breast Cancer. Version 2.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 28 Apr 2022.
Eziokwu AS, Varella L, Kruse ML, Jia X, Moore HCF, Budd GT, et al. Real-world evidence evaluating continuation of CDK4/6 inhibitors beyond first progression in hormone receptor-positive (HR+) metastatic breast cancer. J Clin Oncol. 2019;37(15)suppl):e12538-e. https://doi.org/10.1200/JCO.2019.37.15_suppl.e12538.
Mariotti V, Khong HT, Soliman HH, Costa RL, Fisher S, Boulware D, et al. Efficacy of abemaciclib (abema) after palbociclib (palbo) in patients (pts) with metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):e12521-e. https://doi.org/10.1200/JCO.2019.37.15_suppl.e12521.
Carter GC, Sheffield KM, Gossai A, Huang Y-J, Zhu YE, Bowman L, et al. Abstract P2–08-12: initial real world treatment patterns and outcomes of Abemaciclib for the treatment of HR+, HER2− metastatic breast cancer. Cancer Res. 2020;80(4 Supplement):P2-08–12. https://doi.org/10.1158/1538-7445.Sabcs19-p2-08-12.
Wander SA, Zangardi M, Niemierko A, Kambadakone A, Kim LS, Xi J, et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2− metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1057. https://doi.org/10.1200/JCO.2019.37.15_suppl.1057.
Tamragouri K, Cobleigh MA, Rao RD. Abemaciclib with or without fulvestrant for the treatment of hormone receptor-positive and HER2-negative metastatic breast cancer with disease progression following prior treatment with palbociclib. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.e12533.
Anjos CHD, Razavi P, Herbert J, Colon J, Gill K, Modi S, et al. A large retrospective analysis of CDK 4/6 inhibitor retreatment in ER+ metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1053. https://doi.org/10.1200/JCO.2019.37.15_suppl.1053.
Cuyun Carter G, Sheffield KM, Gossai A, Huang YJ, Zhu YE, Bowman L, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2− metastatic breast cancer. Curr Med Res Opin. 2021;37(7):1179–87. https://doi.org/10.1080/03007995.2021.1923468.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Beyrer J, Manjelievskaia J, Bonafede M, Lenhart G, Nolot S, Haldane D, et al. Validation of an International Classification of Disease, 10th revision coding adaptation for the Charlson Comorbidity Index in United States healthcare claims data. Pharmacoepidemiol Drug Saf. 2021;30(5):582–93. https://doi.org/10.1002/pds.5204.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7. https://doi.org/10.1111/j.1524-4733.2007.00213.x.
Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814–23. https://doi.org/10.1097/00005650-198808000-00007.
Li N, Du EX, Chu L, Peeples M, Xie J, Barghout V, et al. Real-world palbociclib dosing patterns and implications for drug costs in the treatment of HR+/HER2− metastatic breast cancer. Expert Opin Pharmacother. 2017;18(12):1167–78. https://doi.org/10.1080/14656566.2017.1351947.
T Redbook Online™ from Micromedex Solutions®. Truven Health Analytics, Ann Arbor (MI). http://micromedex.com/redbook. Accessed 29 Apr 2022.
Ritzwoller DP, Hassett MJ, Uno H, Cronin AM, Carroll NM, Hornbrook MC, et al. Development, validation, and dissemination of a breast cancer recurrence detection and timing informatics algorithm. J Natl Cancer Inst. 2018;110(3):273–81. https://doi.org/10.1093/jnci/djx200.
Carroll NM, Ritzwoller DP, Banegas MP, O’Keeffe-Rosetti M, Cronin AM, Uno H, et al. Performance of cancer recurrence algorithms after coding scheme switch from international classification of diseases 9th revision to international classification of diseases 10th revision. JCO Clin Cancer Inform. 2019;3:1–9. https://doi.org/10.1200/cci.18.00113.
Huang Y-J, Ryan PD, Price GL, Carter GC, Sheffield KM, Smyth EN, et al. HSR21-051: treatment outcomes among HR+/HER2− advanced/metastatic breast cancer patients receiving CDK 4 & 6 inhibitors in a United States clinical practice setting. J Natl Compr Canc Netw. 2021;19(35): HSR21-051-HSR21. https://doi.org/10.6004/jnccn.2020.7739.
Rassen JA, Schneeweiss S. Newly marketed medications present unique challenges for nonrandomized comparative effectiveness analyses. J Comp Eff Res. 2012;1(2):109–11. https://doi.org/10.2217/cer.12.12.
Balu S, O’Shaughnessy J, Paul ML, Baidoo B, Sudharshan L. EARLY real-world treatment and dosing patterns of ribociclib for metastatic breast cancer (mBC): a retrospective observational study. J Clin Oncol. 2020;38(15_suppl):e13059-e. https://doi.org/10.1200/JCO.2020.38.15_suppl.e13059.
Rugo HS, Huober J, García-Sáenz JA, Masuda N, Sohn JH, Andre VAM, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26(1):e53–65. https://doi.org/10.1002/onco.13531.
Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155–9. https://doi.org/10.5001/omj.2011.38.
Dalal AA, Gagnon-Sanschagrin P, Burne R, Guérin A, Gauthier G, Small T, et al. Dosing patterns and economic burden of palbociclib drug wastage in HR+/HER2− metastatic breast cancer. Adv Ther. 2018;35(6):768–78. https://doi.org/10.1007/s12325-018-0701-5.
Biskupiak J, Oderda G, Brixner D, Tang D, Zacker C, Dalal AA. Quantification of economic impact of drug wastage in oral oncology medications: comparison of 3 methods using palbociclib and ribociclib in advanced or metastatic breast cancer. J Manag Care Spec Pharm. 2019;25(8):859–66. https://doi.org/10.18553/jmcp.2019.25.8.859.
daCosta DM, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–96.
Shah D, Byrd B, Avalos-Reyes E, McCarthy A, Matlin OS, Grover R, et al. Adherence to abemaciclib in patients receiving antidiarrheal medicine. J Clin Oncol. 2021;39(15_suppl):e13016-e. https://doi.org/10.1200/JCO.2021.39.15_suppl.e13016.
Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74. https://doi.org/10.1002/pds.1230.
Gong Y, Kehl KL, Oxnard GR, Khozin S, Mishra-Kalyani PS, Blumenthal GM. Time to treatment discontinuation (TTD) as a pragmatic endpoint in metastatic non-small cell lung cancer (mNSCLC): a pooled analysis of 8 trials. J Clin Oncol. 2018;36(15_suppl):9064. https://doi.org/10.1200/JCO.2018.36.15_suppl.9064.
Gao J, Gong Y, Cheng J, Schroeder R, Amiri-Kordestani L, Khozin S, et al. Abstract P5–14-02: time to treatment discontinuation as a pragmatic endpoint: a U.S. Food and Drug Administration pooled analysis of CDK 4/6 inhibitors. Cancer Res. 2019;79(4 Supplement):P5-14–02. https://doi.org/10.1158/1538-7445.Sabcs18-p5-14-02.
Magdelijns FJ, Stassen PM, Stehouwer CD, Pijpers E. Direct health care costs of hospital admissions due to adverse events in The Netherlands. Eur J Public Health. 2014;24(6):1028–33. https://doi.org/10.1093/eurpub/cku037.
Rodríguez-Monguió R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21(9):623–50. https://doi.org/10.2165/00019053-200321090-00002.
Cardoso F, Wilking N, Bernardini R, Biganzoli L, Espin J, Miikkulainen K, et al. A multi-stakeholder approach in optimising patients’ needs in the benefit assessment process of new metastatic breast cancer treatments. Breast. 2020;52:78–87. https://doi.org/10.1016/j.breast.2020.04.011.
Martín M, Johnston S, Huober J, Di Leo A, Sohn J, Andre VA, et al. 326P-MONARCH 3: updated time to chemotherapy and disease progression following abemaciclib plus aromatase inhibitor (AI) in HR+, HER2− advanced breast cancer (ABC). Ann Oncol. 2019;30:v113–4. https://doi.org/10.1093/annonc/mdz242.021.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–24. https://doi.org/10.1001/jamaoncol.2019.4782.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Eli Lilly & Company.
Conflict of interest
Emily Nash Smyth, Julie Beyrer, Hamed Abedtash, Angelo DeLuca, and Sarah Rybowski are employees and shareholders of Eli Lilly & Company; Garreth W. Lawrence is an employee of Eli Lilly & Company; Kimberly R. Saverno is a former employee and shareholder of Eli Lilly & Company; Elizabeth Hadden is an employee of DeLisle & Associates, Ltd.
Ethics approval
As the databases used in the study are fully de-identified and compliant with the HIPAA, this study was exempt from institutional review board approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
The data that support the findings of this study have been originated by IBM® MarketScan®. These de-identified data are only available through third-party license. As such, the authors cannot make these data publicly available due to data-use agreements. Other researchers can access these data by purchasing a license through IBM Watson Health. Interested individuals may consult https://www.ibm.com/products/marketscan-research-databases for more information on accessing the MarketScan® Research Databases.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. Data analysis was performed by Julie Beyrer and Elizabeth Hadden. All authors drafted, reviewed, and edited the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Smyth, E.N., Beyrer, J., Saverno, K.R. et al. Real-World Patient Characteristics, Utilization Patterns, and Outcomes of US Patients with HR+, HER2− Metastatic Breast Cancer Treated with Abemaciclib. Drugs - Real World Outcomes 9, 681–693 (2022). https://doi.org/10.1007/s40801-022-00327-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00327-1