Abstract
The Cretaceous Mamu Formation coal samples located within the Northern Anambra Basin were collected, analysed and interpreted using multivariate statistical approach to determine the major, trace and rare elements association with a view to determine the source of the terrestrial rocks, palaeoweathering/climatic conditions and tectonic setting. The dominant oxides such as SiO2, Al2O3, Fe2O3, TiO2 and CaO were identified in the coal to suggest terrigenous origin. The ratio of SiO2/Al2O3 of 4.8 suggests that the coal was formed from low land peat associated with freshwater continental marine or blackish water with low salinity as indicated by Sr/Ba and CaO + MgO/K2O + Na2O ratios. The major oxides also revealed stable condition of deposition, low degree of tectonic setting but constant subsidence in the basin. The condition of deposition was acidic in nature as indicated by TiO2/Zr plot. Based on the abundance of Zr, Zn, Ba, Ni, Co, Sr, V, and Y, moderate salinity, sub-oxic to oxic bottom water condition was prevalent and also indication of marine influence. Based on the ratios of La/Yb; La/Sm and Gd/Yb, LREE has higher enrichment than HREE. Humid climatic conditions were observed at the coal formation stage while weak laterization to kaolinization was also evidence.
Similar content being viewed by others
1 Introduction
Coal is a carbon-rich, combustible, stratified organic sedimentary rock composed of altered and/or decomposed plant remains of non-marine origin combined with varying minor amount of inorganic materials (MacDonald et al. 2011; Raistrick 1974). The Anambra Basin, located in the Lower Benue trough is a major coal producing basin in Nigeria. Coal is a complex organic rock comprised of mainly decayed plants conditioned by syngenetic, diagenetic, epigenetic and detrital inorganic elements (Ogala et al. 2010). The Anambra Basin contains important coal measures in the Mamu and Nsukka Formations (Umeji 2005; Uzoegbu et al. 2014). The mining of this sub-bituminous coal occurred in the Mamu Formation. Total coal reserves in the north–south trending coal belt were assed to be about 1.5 billion tons (De Swardt and Cassey 1963). Lignite deposits occur in numerous seams in an east–west belt to the southwest (Simpson 1954; Uzoegbu et al. 2014). A number of studies (Obaje et al. 1999; Reyment 1965; Akande et al. 1992, 2007; Okorie and Egila 2014) have been conducted on the stratigraphic succession, organic and inorganic geochemistry of coal, distribution and abundance of coal in Anambra Basin. Previous work has also been carried out on the trace elements in coal in the study area (Olajire et al. 2007; Ndiokwere et al. 1983; Sonibare et al. 2005; Ewa 2004; Ogala et al. 2009, 2010).
Nigeria is endowed with abundant coal reserves of the required quality suitable for power generation and other by products that are waiting to be tapped (Uzoegbu et al. 2014).
The objectives of this current study are to present the inorganic characteristics of Okaba coal using multivariate statistical approach with a view to determine the oxides/elements association, provenance of terrigenous components of coal deposits, palaeoclimate/weathering environments and tectonic settings.
2 Geological setting
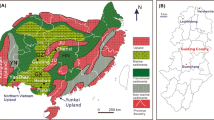
Anambra basin, located in the Lower Benue Trough is a major coal producing basin in Nigeria (Fig. 1). Anambra basin, a near triangular basin is located in eastern part of Nigeria. The basin is bounded in the west by the Okitipupa ridge, in the south by the Niger Delta Basin, to the northwest it directly overlies the Basement complex and inter fingers the Bida Basin (Adebayo et al. 2015; Osazuwa and Saka 2016). Anambra basin lies between longitudes 6.3 °E and 8.0 °E and latitudes 5.0 °N and 8.0 °N. This basin is delimited in the north by the Basement complex, the Middle Benue Trough and the Abakaliki Anticlinorium. Authors such as Akande and Erdtmann (1998), Obaje et al. (1999) have considered the basin as the lower Benue Trough, a NE–SW trending, folded, aborted rift basin that runs obliquely across Nigeria. Its origin was linked to the tectonic processes that accompanied the separation of the African and South American plates in the Early Cretaceous (Reyment and Tait 1972). This rift model is supported by evidence from structural, geomorphic, stratigraphic and paleontological studies (Reyment 1969; Burk et al. 1972; Murat 1972). The evolution of Anambra Basin represents the third cycle in the evolution of the trough and its associated basins when the Abakaliki Trough was uplifted to form the Abakaliki Anticlinorium while the Anambra platform was downwarped to form the Anambra Basin resulting in the westward displacement of the trough’s depositional axis (Murat 1972; Webber and Daukoru 1975; Adebayo et al. 2015).
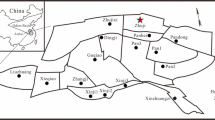
General geology of Nigeria (after Obaje et al. 1994)
The basin is a sedimentary succession that directly overlies the facies of the Lower Benue trough and consists of Campanian to early Palaeocenelithofacies (Adebayo et al. 2015). Several works on the Palaeoenvironment, palaeogeography, sedimentary tectonics, coal deposits and lithostratigraphy have been carried out (Nwajide and Reijers 1996; Akandeet al. 2007; Ogala et al. 2009, 2010; Onyekuru and Iwuagwu 2010; Okorie and Egila 2014; Akinyemi et al. 2014; Uzoegbu et al. 2014; Adebayo et al. 2015). Sediments deposition within the Anambra Basin started during the Campanian, with Nkporo and Enugeshales constituting the basal beds of the Campanian period. The Campanion was a period of short marine transgression and regression, where the shallow-sea later became shallower due to subsidence (Akinyemi et al. 2014). This resulted in a regressive phase during the Maastrichtian which deposited the flood plain sediments and deltaic foresets of Mamu Formation regarded as the Lower coal measures. The Mamu Formation is overlain by the Ajali sandstones which also overlain by Nsukka Formation (Akinyemi et al. 2014).
2.1 Lithostratigraphy of study area
-
(1)
Nkporo Formation This is the basal sedimentary unit deposited during the Santonian folding and inversion in south eastern Nigeria and indicates a late Campanian age (Odumoso et al. 2013). The formation is poorly exposed but has been described as coarsening upward deltaic sequence of shale and interbed of sands and shale with occasional thin beds of limestones (Odumoso et al. 2013).
-
(2)
Enugu shales This consists of carbonaceous grey black shales and coals with interbeds of very fine sandstones/siltstones deposited in lower flood plain and swampy environment (Fig. 2). The bedding planes are poorly defined with early diagenetic minerals such as pyrite and siderites. The Enugu shale is assigned Campanian to Lower Maastrichtian (Odumoso et al. 2013).
-
(3)
Mamu Formation This formation overlies the Enugu shales conformably and contains sandstones, shale, mudstone, sandy-shale with coal seams in various horizons. The sediment pile ranges from 75 to over 100 m (Fig. 2). These deposits are estuarine flood plain, swamp and flat flood (Odumoso et al. 2013).
-
(4)
Ajali Sandstones This Formation (Middle Maastrichtian) overlies the Mamu Formation and has Middle to Upper Maastrichtian age (Fig. 2). It has thickness range of less than 300 m to over 1000 m at the Centre of the basin. This formation is made up of texturally mature sand facies (Odumoso et al. 2013).
-
(5)
Nsukka Formation The Nsukka Formation is Upper Maastrichtian to Danian in age. It consists of alternating sandstones, sandy shales and mudstones with interbedded coal seams (Fig. 2). The marine shales of the Nsukka Formation were deposited in the Palaeocene, overlain by the tidal Nanka sandstones of Eocene age which constitute the Tertiary succession (Ogala et al. 2010).
3 Materials and methods
Eighteen samples were collected from six locations scattered around Kogi east, Northern Anambra Basin, Nigeria (Fig. 3). These eighteen representative samples were pre-prepared by washing, cutting and bagging and sent to Acme Laboratory Canada for sample preparation and analysis. All samples were analyzed at the Acme Analytical Laboratories Ltd, Canada for major, minor and trace element geochemistry. The ICP-mass spectrometer (Perkin-Elmer, Elan 6000) and inductively coupled plasma-mass spectrograph were used for the analysis. The samples were digested by weighing 0.2 g aliquot in a graphite crucible mixed with 1.5 g Lithium metaborate/tetraborate (LiBO2/LiB4O7) to form flux. The crucibles were placed in an oven and heated up to 980 °C for 30 min. The cooled bead was dissolved in 5% HNO3 (ACS grade nitric aciddiluted in demineralized water). Calibration standards and reagent blankswere added to sample sequences. The basic package that consists of thirty-fourelements (Ba, Co, Cs, Ga, Hf, Nb, Rb, Sn, Sr, Ta, Th, U, V, Y, Zr, La, Ce, Pr, Nd, Sm, Eu, Gd, Lu) was determined. A second 0.5 g split sample was digested in Aqua Regia and analysedby ICP-MS to determine Au, Ag, As, Bi, Cd, Cu, Hg, Mo, Ni, Pb, Sb, Seand Zn. An ICP-emission spectrograph (SpectroCiros Vision or Varian 735)was also used for determination of major oxides and some trace elements (SiO2, Al2O3, Fe2O3, CaO, MgO, TiO2, P2O5, Cr2O5, Ba, Nb, Ni, Sr, Sc, Y and Zr). Loss on ignition (LOI) was determined by the weight loss upon heating 1 g split sample at 95 °C for 90 min.
Multivariate technique was applied to reveal hidden affinities within the database and undetectable by other means (Kim et al. 2003). This method helps in extracting information about the similarities or dissimilarities among parameters. Latent factors in data sets were identified and variables responsible for the variations observed explained the structure of the data set (Kim et al. 2003). This is the basis for factor and cluster analyses which themselves are based on correlation coefficient relationships.
Factor analysis through a linear dependence model constructed in an abstract space called factor score space was used. It searches for correlations among measured variables that characterized a set of geochemical data (Pathak et al. 2008). Factor analysis reduces space dimensionality to obtain a better grouping of variables and determine relations between major components and variables analysed (Ameh et al. 2014). Elements association obtained from factor analysis is mathematically independent. The factor solutions provide information on loadings; communalities and eigenvalues (Lokhande et al. 2008).
Cluster analysis was also performed to classify elements of different geochemical sources on the basis of similar chemical properties. Hierarchical cluster analysis was used to find the true group of data. Cluster analysis using dendograms was used to identify relatively homogeneous groups of variables with similar properties (Pravenna et al. 2007). Each cluster group shows similar and specific groups with respect to source and geochemical characteristics. Data for cluster analysis were standardized to equalize the influence of the geochemical variables.
4 Results and discussion
-
(1)
Evidence from major oxides
The oxides, trace and rare elements are present in coals in inorganic forms. Many elements in coal are derived from the detrital inputs to the precursor swamp and many chalcophile elements associated with sulphide minerals (Ogala et al. 2010). The inorganic elements present in the coals suggest that these elements were sourced from terrigenous origin (Yandoka et al. 2015).
The result of the dominant coal major oxides shows average mean weight% of SiO2 (16.36); Al2O3 (3.5); Fe2O3 (1.20); TiO2 (0.44) and CaO (0.14) respectively. The rest oxides have significantly low mean weight percentages. The percentage weight of LOI is 78.06 (Table 1). The low Al2O3 could imply limited supply of detrital materials in peat and low level of weathering in source area and coal seams (Stanislav et al. 2010). On the basis of the concentrations of CaO; Na2O and MgO, this coal may have been formed under continental freshwater conditions and low salinity environment. The major source of K in coal could be detrital, from coal intrusive and volcanic rocks which were clearly absent here. Also, the low Ti concentration observed may be due to limited supply of Ti in peat and high in situ nature of inorganic matter (Stanislav et al. 2010).
-
(2)
Coal oxides ratios
The SiO2/Al2O3 ratio recorded is 4.58. The low SiO2/Al2O3 ratio is an indication that the coal was formed under stable conditions of deposition, where subsidence was slow and steady and with low degree of tectonic activities (Yossifova et al. 2009; Zhao et al. 2015; Stanislav et al. 2010).
The CaO + MgO/K2O + Na2O and the ratio of CaO/MgO revealed values of 4.25 and 4.67 respectively. These ratios shows that the coals were probably associated with marine or brackish water influences, saline lakes or inorganic matter enriched in algal remains (Stanislav et al. 2010).
According to Chen et al. (2016), Al2O3 and TiO2 in source rocks are preserved in the clastic sedimentary rocks because Al and Ti are not readily mobilized by weathering processes. The ratio of Al2O3/TiO2 in sandstones, mudstones and other sedimentary rocks changed insignificantly during weathering of source rocks and subsequent transportation, deposition and diagenesis of the sediments (Hayashi et al. 1997).
Based on the discriminating criterion used to distinguished different types of parent igneous rocks, the ratio of Al2O3/TiO2 in the study coal ranged from 3 to 10 (Fig. 4). This reflects predominantly basalt to granite rocks as the source of the terrestrial sediments that form part of the coal (Amajor 1987; Chen et al. 2016).
Provenance diagram of Al2O3 versus TiO2 in coal (after Amajor 1987)
-
(3)
Evidence from major and trace elements
The plot of TiO2 versus Zr can be compared with earlier defined source rock fields (Stone et al. 1987; Hayashi et al. 1997). From the TiO2 versus Zr plot (Fig. 5), the inorganic components of the coal around the study area falls completely in the intermediate igneous rocks. This plot, like the earlier one showed that the terrestrial source of sediments were predominantly from intermediate igneous rocks.
Provenance diagram of TiO2 versus Zr in study area (after Hayashi et al. 1997)
The provenance plot of TiO2/Ni (Fig. 6) revealed that most samples plotted within the acidic field while only three samples plotted around the sandstones sedimentary trend. This is consistent with earlier plots that were predominantly basalt-granite source for the sediments.
Provenance diagram of TiO2 versus Ni in coal samples (Floyd et al. 1989)
-
(4)
Major oxides correlation
Given P < 0.01 (Table 2), analysis of major oxides of coal revealed strong correlation between SiO2 and TiO2 (0.999); SiO2 and LOI (0.998); Al2O3 and TiO2 (0.921); Al2O3 and Cr2O3 (0.962); Al2O3 and LOI (0.932); CaO and MnO (0.927); TiO2 and Cr2O3 (0.964); TiO2 and LOI (−0.999); Cr2O3 and LOI (−0.969). At P < 0.05, significant correlations were also observed between SiO2 and Al2O3 (0.907); SiO2 and P2O5 (0.956); SiO2 and Cr2O3 (0.958); Al2O3 and Na2O (0.887); MgO and MnO (0.813); TiO2 and P2O5 (0.956); P2O5 and Cr2O3(0.958); P2O5 and LOI (−0.948).
The dominant major oxides were SiO2; Al2O3 and Fe2O3. The likely carriers of these elements were quartz; clay minerals and pyrites (Zhao et al. 2015). The relatively high presence of TiO2 is an indication that Ti was incorporated into the aluminosilicates structure. It could also implied that kaolinite and TiO2 co-precipitated or that the TiO2 content could be as a result of the following: that the Ti was incorporated into the aluminosilicates structure; that kaolinite and TiO2 co-precipitated or that the Ti content from the intermediate rocks were probably high (Zhao et al. 2015). The high correlation between CaO–MnO (0.927) and MgO–MnO (0.813) shows that the CaO and MgO in the coals maybe associated with carbonates (calcite, dolomite and ankerite). From Table 2, TiO2; Cr2O3; LOI and Na2O, all showed positive correlation with Al2O3. This suggests that these oxides in coal were associated with aluminosilicates such as clay minerals and probably from the same source (Zhao et al. 2015). The Cr2O3 and LOI showed positive relationship with P2O5. This affinity implies aluminophosphates minerals (Zhao et al. 2015).
Two clusters were extracted from the oxides of coal analysis. Cluster one consist of SiO2, TiO2, P2O5, Cr2O3, Al2O3, K2O, MgO and Na2O. Within this cluster, strongest similarities were observed between SiO2, TiO2, Cr2O3, K2O and Na2O. A lesser degree of similarities were observed within same cluster between P2O5 and Al2O3. Cluster two includes: CaO, MnO, Fe2O3, and LOI. Most significant similarity was however observed between MnO and LOI (Fig. 7).
While cluster one indicates mixtures of origin such as detrital quartz, clay minerals, basaltic salts of oceanic environment and some degree of carbonate affinity, cluster two suggests carbonate affinity and Fe bearing montmorillonite, chlorite and oxyhydroxides sources (Stanislav et al. 2010; Taghipour and Marshk 2015).
Varimax rotated analysis extracted two factors as responsible for the 100% cumulative variance observed in the data (Table 3). Factor one consist of high factor loadings for SiO2 (0.986); Al2O3 (0.972); Fe2O3 (−0.999); MgO (0.538); Na2O (0.729); K2O (0.929); TiO2 (0.989); P2O5 (0.957); and LOI (−0.992). This factor has eigenvalue of 8.685 and % variance of 72.378. Factor two include high factor loadings of MgO (0.843); CaO (0.941); Na2O (0.684); MnO (0.974). Eigenvalue of 3.315 and % variance of 27.622 was recorded.
From the rotated result (Table 3), component two revealed high factor loadings for all variables except K2O (0.684). Theses high factor loadings suggest lignites while the relatively low K2O could imply a higher ranking coal (Stanislav et al. 2010). Component one ranged from factors such as aluminosilicates, aluminophosphates and basalts as possible sources for the variables (Zhao et al. 2015).
-
(5)
Trace Elements
The geochemical behavior of trace and rare elements in modern, organic rich sedimentary rocks such as coal has been documented and used to determine the terrestrial sediments source component of the organic rocks (Adebayo et al. 2015).
The trace element analysis (Table 4) indicates abundance order of: Zr > Zn > Ba > V> As > Y > Cu > Ni > Co > Sr > Ga > Be > Hf > Pb > Th > Nb > Sc > Sn. The ratio Sr/Ba of 0.176 showed that these coal occurrences were deposited on the continent. The V/(V + Ni) ratio of 0.66 is also considered as transitional boundary fromoxic to suboxic and anoxic depositional environment (Taghipour and Marshk 2015).
The correlation (Table 5) revealed that the likely source of the trace elements was lithophilic at P = 0.01. While significant relationship was recorded between the lithophiles and the chalcophiles from trace elements such as Nb to W, it is worthy to state that these relationship occurred at P = 0.005. At P = 0.001 and P = 0.05, from trace element Y to Sb, the chalcophiledominated the association (Table 5).
The trace element yielded four clusters (Fig. 8). Cluster one (Bi, Au and Cd) shows that all the elements were lithophilic. Cluster two is a mixture of sources. From dominantly lithophilic (Sc, V, Y, Cu and Be) to chalcophilic (Ga, Hg and Tl) and finally the siderophile (Mo). Cluster three suggests three sources for the elements—the siderophile (Zn, Sb, Ni, Co and Se), the lithophiles (Ba, Sr) and the chalcophiles (As). The last cluster is a mixture of two sources. These are lithophilic (Rb, Hf, Zr, Nb, Th, U, Ta, Cs and W) and the Chalcophile (Pb, Sn and Ag).
From above analysis, it is clear that the dominant sources for the trace elements were the lithophiles (clusters 4 and 2); Siderophile (clusters 3 and 2) and the chalcophile (clusters 1, 2, 4 and 3).
-
(6)
Rare Earth Elements
The LREE generally have higher mean concentration compared to the HREE (Table 6). The ratios of La/Yb (5.77); La/Sm (2.82) and Gd/Yb (2.33) showed that the degree of enrichment of LREE is higher than HREE. This is an evidence that fractionation occurred in the LREE and HREE. The average ratio (La/Sm) n value of 2.82 indicate that some degree of fractionation also occurred among the LREE (Taghipour and Marshk 2015).
At P < 0.01, all the correlations recorded between Sm–Eu–Gd–Tb–Dy–Ho–Er–Lu–Tm–Yb were significant (Table 7) in both directions. Ce showed no significant relationship with any element. La also showed no significant relationship with all the elements except La and Ce (0.897). From Table 7, the REEs appeared to be generally related to primarily clay minerals and phosphates except La and Ce that may suggests dominantly phosphatic affinity with organic origin.
Cluster analysis of rare earth elements yielded two clusters (Fig. 9). Cluster one consists of Tb, Dy, Gd, Ho, Er, Eu, Sm, Tm, Yb and Lu. Most significant similarities exist between Tb–Dy–Gd–Ho–Eu–Sm–Tm and Lu. Between Er and Yb was a lesser degree of similarity. Cluster two consists of La and Ce. These two elements showed the most significant similarity (Fig. 9).
From Fig. 9 and Table 7, La and Ce appeared in the same cluster, factor component and also showed very strong correlation (0.987). This is an indication that these elements were from same source and are controlled by factor (s) different from other rare earth elements appeared in the same cluster and factor component. Other REEs such as Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu maybe associated with the inorganic component of the coal (Zhao et al. 2015).
The rare earth element analysis recorded two factor components (Table 8). Factor one consists of high factor loadings of 0.8–0.9 for all rare earth elements analysed except La and Ce. This factor has eigenvalue of 9.350 and variance of 77.920%. Factor two consists of high factor loadings of La (0.967) and Ce (0.986) only. The cumulative of 99.167% was enough for the variations observed (Table 8).
-
(7)
Palaeoweathering/climatic conditions of deposition
According to Suttner and Dutta (1986), binary plot of SiO2 versus (Al2O3 + K2O + Na2O) has been used to reconstruct the climatic conditions of the source area. Based on this plot, the coal samples from the study area plotted within the humid field (Fig. 10). This indicates that both weathering of source rocks/materials and conditions surrounding the coal formations occurred in humid climatic environments (Chen et al. 2016; Schellmann 1981).
Paleoclimatic diagram of SiO2 versus Al2O3 + K2O + Na2O (AFTER Suttner and Dutta 1986)
The Si becomes unstable in rocks due to removal of silica rocks as weathering progresses. According to Chen et al. 2016, the SiO2–Al2O3–TFe2O3 (SAF) ternary diagram proposed by Schellmann (1986) can be used to quantify the degree of laterization. On the basis of the SAF ternary diagram above, the coal samples from the study area may have suffered weak laterization to kaolinisation (Fig. 11).
Triangular diagram of SiO2–Al2O3–Fe2O3 (after Chen et al. 2016)
5 Conclusion
-
(1)
The coal was formed from low land peat environment associated with marine or blackish water influence.
-
(2)
Stable condition of deposition, low degree of tectonic movement but constant subsidence in the basin was observed.
-
(3)
Transitional boundary of oxic to sub-oxic and anoxic environments were observed with acidic and humid climate.
-
(4)
Weak laterization to kaolinization were also observed.
References
Adebayo OF, Akinyemi SA, Ojo AO (2015) Palaeoenvironmental studies of Odagbo coal mine sequence, Northern Anambra Basin, Nigeria: insight from palynomorph and geochemical analyses. Int J Curr Res 7(09):20274–20286
Akande SO, Erdtmann BD (1998) Burial metamorphism (maturation) in Cretaceous sediments of the southern Benue Trough and the Anambra Basin, Nigeria. Am Assoc Pet Geol Bull 82:1191–1206
Akande SO, Hoffinecht A, Erdtmann BD (1992) Upper cretaceous and tertiary coals from southern Nigeria. Composition, rank, depositional environments and their technological properties. NAPE Bull 7:26–38
Akande SO, Ogunmoyero IB, Petersen HI, Nytoft HP (2007) Source rock evaluation of coals from the Lower Maastrichtian Mamu Formation, SE Nigeria. J Pet Geol 30(40):303–324
Akinyemi SA, Adebayo OF, Ojo AO, Fadipe AO, Gitari WM (2014) Geochemistry and mineralogy of campanian sandstone of Lokoja-Basange formation, middle Niger Basin (Lokoja sub-basin), Nigeria: implications for provenance, weathering, tectonic setting and paleo-redox condition. J Nat Sci Res 4(16):65–89
Amajor LC (1987) Major and trace element geochemistry of Albian and Turonianshales from the Southern Benue Trough Nigeria. J Afr Earth Sci 6:633–641
Ameh EG, Imasuen IO, Imeokparia EG (2014) Geochemistry and environmentic approaches to estimate pollution impacts on soils around Okaba coal mines. British J Appl Sci Technol 4(17):2513–2527
Burke KC, Dessauvagie TFJ, Whiteman AJ (1972) Geologic history of the Benue Valley and adjacent areas. In: Dessauvagie TFJ, Whiteman AJ (eds) African geology. Univ. Ibadan Press, Ibadan, pp 187–206
Chen B, Liu G, Wu D, Sun R (2016) Comparative study on geochemical characterization of the Carboniferous aluminous argillites from the Huainan Coal Basin, China. Turk J Earth Sci 25:274–287
De Swardt AJ, Cassey OP (1963) The coal resources of Nigeria. Geol Surv Niger Bull 28:100
Ewa IOB (2004) Data evaluation of trace elements determined in Nigerian coal using cluster procedures. Appl Radiat Isot 60(5):751–758
Floyd PA, Winchester JA, Park RG (1989) Geochemistry and tectonic setting of Lewisian clastic metasediments from the early Proterozoic Loch Maree group of Gairloch, NW Scotland. Precambrian Res 45:203–214
Hayashi K, Fujisawa H, Holland H, Ohmoto H (1997) Geochemistry of approx. 1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim Cosmochim Acta 61(19):4115–4137
Kim J-H, Yum B-W, Kim R-H, Koh D-C, Koh D-C, Cheong T-J, Lee J, Chang H-W (2003) Application of cluster analysis for the hydrogeochemical factors of saline groundwater in Kimje, Korea. Geosci J 7(4):313–322
Lokhande PB, Patit VV, Mujawar HA (2008) Multivariate statistical analysis of groundwater in the vicinity of Mahad industrial area of Konkan region, India. Int J Appl Environ Sci 3(2):149–163
MacDolnald JG, Burton CJ,Winstanley I, Lapidus DF (2011) Collins internet-linked dictionary of geology. Learning Solutions Specialty Publications Ltd., an imprint of Rombic Concepts Ltd. pp 480–484
Murat RC (1972) Stratigraphy and paleogeography of the cretaceous and lower tertiary in Southern Nigeria. In: Dessauvagie TFJ, Whiteman AJ (eds) African geology. Ibadan University Press, Ibadan, 1972, pp 251–266
Ndiokwere CL, Guinn VP, Burtner D (1983) Trace elemental composition of Nigerian coalmeasured by neutron activation analysis. J Radioanal Nucl Chem 79(1):123–128
Nwajide CS, Reijers TJA (1996) Sequence architecture in outcrops: examples from the Anambra Basin, Nigeria. Niger Assoc Pet Explor Bull 11(1):23–32
Obaje NG, Ligouls B, Abas SI (1994) Petrographic composition and depositional environments of Cretaceous coals and coal measures in the Middle Benue Trough of Nigeria. Int J Coal Geol 26:244–260
Obaje NG, Ulu OK, Petters SW (1999) Biostratigraphy and geochemical controlsof hydrocarbon prospects in the Benue Trough and Anambra Basin, Nigeria. NAPE Bull 14:18–54
Odumoso SE, Oloto IN, Omoboriowo AO (2013) Sedimentological and depositional enviroment of the Mid-MaastritchtianAjali Sandstone, Anambra Basin, Southern Nigeria. Int J Sci Technol 3(1):2224–3577
Ogala JE, Akaegbobi MI, Omo-Irabor OO, Finkelman RB (2009) Statistical analysis of geochemical distribution of major and trace elements of the Maastrichtian coal measures in the Anambra Basin. Nigeria. Petroleum coal 51(4):260–269
Ogala JE, Omo-Irabo OO, Finekleman RB, Akaegbobi I (2010) Major oxides and trace element distribution in coal and coaly shale seams in the Enugu escarpment of South-Eastern Nigeria. Global J Geol Sci 8(4):175–186
Okorie EO, Egila JN (2014) Trace and major metal abundances in the shale and coal of various seams at Okaba coal mine, Kogi State, Nigeria. Int J Biol Chem Sci 8(2):741–754
Olajire AA, Ameen AB, Abdul-Hammed M, Adekola FA (2007) Occurrence anddistribution of metals and porphyrins in Nigericoal minerals. J Fuel Chem Technol 35(6):641–647
Onyekuru SO, Iwuagwu CJ (2010) Depositional environments and sequence stratigraphic interpretation of the Campano-Maastrichtian Nkporo Shale Group and Mamu Formation Exposures at Leru-Okigwe Axis, Anambra Basin, Southeastern Nigeria. Aust J Basic Appl Sci 4(12):6623–6640
Osazuwa AO, Saka AO (2016) Provenance of sandstone on the western flank of Anambra Basin, Southwestern Nigeria. Glob J Geol Sci 14:13–21
Pathak JK, Mohd Alam, Shikha Sharma (2008) Interpretation of groundwater quality using multivariate statistical technique in Moradabad City, Western Uttar Pradesh State, India. J Chem 5(3):607–619
Praveena SM, Ahmed A, Radojevic M, Abdullah MH, Aris AZ (2007) Factor-cluster analysis and enrichment study of mangrove sediments—an example from Mengkabong, Sabah. Malays J Anal Sci 11(2):421–430
Raistrick A (1974) Teach yourself geology. English University Press Ltd., London, pp 241–243
Reyment RA (1965) Aspects of the geology of Nigeria. University of Ibadan, Ibadan, p 145p
Reyment RA (1969) Ammonite biostratigraphy, continental drift and sscillatory transgressions. Nature 224:137–140
Reyment RA, Tait EA (1972) Biostratigraphical dating of the early history of the South Atlantic. Philos Trans R Soc Lond B264:55–95
Schellmann W (1981) Considerations on the definition and classification of laterites. A critique of the Schellmann definition and classification of laterite. CATENA 47:117–131
Schellmann W (1986) A new definition of Laterite. Mem Geol Surv India 120:1–7
Simpson A (1954) The Nigerian coalfield. The geology of parts of Onitsha, Owerri and Benue Provinces. Bull Geol Surv Niger 24:1–67
Sonibare OO, Ehinola OA, Egashira R, Lim K (2005) An investigation into the thermal decomposition of Nigerian coal. J Appl Sci 5(1):104–107
Stone WE, Jensen LS, Church WR (1987) Petrography and geochemistry of an unusual Fe-rich basaltic komatiite from Boston Township, northeastern Ontario. Can J Earth Sci 24:2537–2550
Suttner LJ, Dutta PK (1986) Alluvial sandstone composition and Paleoclimate. I. Framework mineralogy. J Sediment Pet 56:329–345
Taghipour N, Marshk ZS (2015) Geochemistry and origin of elements of Upper Triassic Olang coal deposits in Northeastern Iran. Geopersia 5(2):189–204
Umeji OP (2005) Palynological study of the Okaba coal mine section in the Anambra Basin, Southern Nigeria. J Min Geol 41(2):193–203
Uzoegbu UM, Ekeleme IA, Uchebo UA (2014) Oil generation capacity of Maastrichtian coals from the Anambra Basin, South eastern Nigeria. Int J Eng Sci 3(4):33–46
Vassilev SV, Vassileva CG, Baxter D, Andersen LK (2010) The relationship between chemical and mineral composition of coal and their potential applications as genetic indicators. Geol Balc 39(3):21–41
Weber KJ, Daukoru EM (1975) Petroleum geological aspects of the Niger Delta. J Min Geol 12:9–22
Yandoka BMS, Abdullah WH, Abubakar MB, Hakimi MH, Adegoke AK (2015) Geochemistry of the Cretaceous coals from Lamja Formation, Yola Sub-basin, Northern Benue Trough, NE Nigeria: implications for paleoenvironment, paleoclimate and tectonic setting. J Afr Earth Sci 104(2015):56–70
Yossifova M, Lerouge C, Deschamps Y (2009) Mineral matter and trace elements in the Vulche pole coal, Bulgaria. Geolines 22:89–94
Zhao L, Wqrd CR, French D, Graham IT (2015) Major and trace element geochemistry of coals and intra-seam claystones from the Songzao Coalfield, SW China. Minerals 5:870–893
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ameh, E.G. Geochemistry and multivariate statistical evaluation of major oxides, trace and rare earth elements in coal occurrences and deposits around Kogi east, Northern Anambra Basin, Nigeria. Int J Coal Sci Technol 6, 260–273 (2019). https://doi.org/10.1007/s40789-019-0247-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-019-0247-4