Abstract
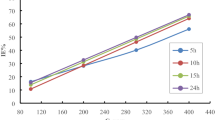
The corrosion inhibition of aluminium in 1 M H2SO4 using expired drugs of moxifloxacin and betnesol as corrosion inhibitors has studied by weight loss measurements, potentiodynamic polarization studies, electrochemical impedance spectroscopy and scanning electron microscope analysis. The experimental studies show that drugs thus used inhibitors reduce the corrosion of aluminium and the inhibition efficiency increases with addition of moxifloxacin and betnesol at various temperatures. Moreover, potentiodynamic polarization curves illustrate that both betnesol and moxifloxacin act as mixed type inhibitors but predominantly anodic process of corrosion. In addition, EIS studies show that transfer resistance increases with the addition of inhibitor concentration. The effect of temperature on aluminium corrosion upon with and without the presence of inhibitors was also investigated in detail. Thermodynamic and adsorption isotherm calculations reveal that the used drug molecules have both chemisorption and physisorption with aluminium metal and followed the Langmuir adsorption isotherm.
Graphical Abstract












Similar content being viewed by others
References
Addel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Kinetics and thermodynamics of aluminium dissolution in 1.0 M sulphuric acid containing chloride ions. Mater Chem Phys 98:291–297
Bai CY, Chou YH, Chao CL, Lee SJ, Ger MD (2008) Surface modifications of aluminum alloy 5052 for bipolar plates using an electroless deposition process. J Pow Sou 183:174–181
Lorking KF, Mayne JEO (1961) The corrosion of aluminium. J Appl Chem 11:170–180
Winston Revie R (2011) Uhlig’s corrosion handbook, 3rd edn. Willey, New York
Ono S, Habazaki H (2009) Effect of sulfuric acid on pit propagation behaviour of aluminium under AC etch process. Corros Sci 51:2364–2370
Zhang JS, Zhao XH, Zuo Y, Xiong P (2008) The bonding strength and corrosion resistance of aluminum alloy by anodizing treatment in a phosphoric acid modified boric acid/sulfuric acid bath. J Surf Coat Tech 202:3149–3156
Garrigues L, Pebere N, Dabosi F (1996) An investigation of the corrosion inhibition of pure aluminum in neutral and acidic chloride solutions. Electrochim Acta 41:1209–1215
Bregmann II (1963) Corrosion inhibitors. Macmillan, New York
Nathan CC (1977) Organic inhibitors. NACE, Houston
Domingue L, Fernandes JCS (2003) Anodising of Al 2024-T3 in a modified sulphuric acid/boric acid bath for aeronautical applications. Corros Sci 45:149–160
Liu Y, Sun D (2005) Corrosion resistance properties of organic–inorganic hybrid coatings on 2024 aluminum alloy. Appl Surf Sci 246:82–89
Na KH, Pyun SI (2006) Effect of sulphate and molybdate ions on pitting corrosion of aluminium by using electrochemical noise analysis. J Electroanal Chem 596:7–12
Lundvall O, Gulppi M (2007) Copper modified chitosan for protection of AA-2024. Surf Coat Technol 201:5973–5978
Niknahad M, Moradian S (2010) The adhesion properties and corrosion performance of differently pretreated epoxy coatings on an aluminium alloy. Corros Sci 52:1948–1957
Harvey TG (2013) Cerium-based conversion coatings on aluminium alloys: a process review. Corros Eng Sci Technol 48:248269
Golru SS, Attar MM (2015) Effects of different surface cleaning procedures on the superficial morphology and the adhesive strength of epoxy coating on aluminium alloy 1050. Prog Org Coat 87:52–60
Clubby BG (1990) Chemical inhibitors for corrosion control. R Soc Chem, Cambridge, p 141
Sputnik E, Ademovic Z, Lisac (1995) Proceedings of the 8th European symposium on corrosion inhibitors (8SEIC), Ann. Univ. Ferrara, NS, Sez V, Suppl 257
Saidman SB, Bessone JB (2002) Electrochemical preparation and characterisation of polypyrrole on aluminium in aqueous solution. J Electroanal Chem 521:87–94
Gomma GK, Wahdan MH (1995) Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater Chem Phys 39:209–213
Ashassi-Sorkhabi H, Shabani B, Aligholipour B, Seifzadeh D (2006) The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl Surf Sci 252:4039–4047
Fares MM, Maayta AK, Al-Mustafa JA (2012) Corrosion inhibition of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros Sci 65:223–230
Nathiya RS, Raj V (2017) Evaluation of Dryopteris cochleata leaf extracts as green inhibitor for corrosion of aluminium in 1 M H2SO4. Egypt J Pet 26:313–323
Nathiya RS, Perumal Suresh, Vajjiravel Murugesan PM, Anbarasan V Raj (2017) Agarose as an efficient inhibitor for aluminium corrosion in acidic medium: an experimental and theoretical study. J Bio Tribo Corros 3:44
Gokhan G (2011) Drugs: a review of promising novel corrosion inhibitors. Corros Sci 53:3873–3898
Xue Hui P, Wen Juan G, Wei Hua L, Jian Dong X, Bao Rong H (2008) Electrochemical, quantum chemical and SEM investigation of the inhibiting effect and mechanism of ciprofloxacin, norfloxacin and ofloxacin on the corrosion for mild steel in hydrochloric acid. Sci China Ser B Chem 51:928–936
Singh AK, Quraishi MA (2010) Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros Sci 52:152–160
Shukla SK, Quraishi MA (2010) Cefalexin drug: a new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Mater Chem Phys 120:142–147
Fouda AS, Abo-Elmeaty WM, El-Abbasy HM (2009) Inhibitive action of some 3-thiazinonyl-bicyclo[4.2.0] octene-carboxylate derivatives drugs on the corrosion of SS type 304 in 1 M HCl solution. Zaštita Mater 50:3–11
Fouda AS, Mostafa HA, El-Abbasy HM (2010) Antibacterial drugs as inhibitors for the corrosion of stainless steel type 304 in HCl solution. J Appl Electrochem 40:163–173
Dubey RS, Potdar Y (2009) Corrosion inhibition of 304 stainless steel in sodium chloride by ciprofloxacin and norfloxacin. J Chem Tech 16:334–338
Pang X, Ran X, Kuang F, Xie J, Hou B (2010) Inhibiting effect of ciprofloxacin, norfloxacin and ofloxacin on corrosion of mild steel in hydrochloric acid. Chin J Chem Eng 18:337–345
Eddy NO, Stoyanov SR, Ebenso EE (2010) Fluoroquinolones as corrosion inhibitors for mild steel in acidic medium; experimental and theoretical studies. Int J Electrochem Sci 5:1127–1150
Eddy NO, Ibok UJ, Ebenso EE, El Nemr A, El Ashry ESH (2009) Quantum chemical study of the inhibition of the corrosion of mild steel in H2SO4 by some antibiotics. J Mol Mod 15:1085–1092
El-Naggar MM (2007) Corrosion inhibition of mild steel in acidic medium by some sulfa drugs compounds. Corros Sci 49:2226–2236
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52:198–204
Abdallah M (2004) Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros Sci 46:1981–1996
Obot IB, Obi-Egbedi NO, Umoren SA (2009) Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros Sci 51:1868–1875
Mourya P, Banerjee S, Rastogi RB, Singh MM (2013) Inhibition of mild steel corrosion in hydrochloric and sulfuric acid media using a thiosemicarbazone derivative. Ind Eng Chem Res 52:12733–12747
Negma NA, Kandile NG, Aiad IA, Mohammad MA (2011) New eco-friendly cationic surfactants: synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surf A Physicochem Eng Asp 391:224–233
Singh A, Lin Y, Eno Ebenso E, Liu W, Pan Jie, Huang Bo (2015) Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J Ind Eng Chem 24:219–228
Mobin Marziya Rizvi M (2017) Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1 M HCl solution. Carbohydr Polym 160:172–183
Gomma GK (1998) Corrosion of low-carbon steel in sulphuric acid solution in presence of pyrazole—halides mixture. Mater Chem Phys 55:241–246
Oguzie EE, Okolue BN, Ebenso EE, Onuoha GN, Onuchukwu AI (2004) Evaluation of the inhibitory effect of methylene blue dye on the corrosion of aluminium in hydrochloric acid. Mater Chem Phys 87:394–401
Ebenso EE (2003) Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine. Mater Chem Phys 79:58–70
Aramaki K, Hackerman M (1969) Inhibition mechanism of medium-sized polymethyleneimine. J Electrochem Soc 116:568–574
Denga S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415
Ahamad Prasad R, Quraisi MA (2010) Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros Sci 52:1472–1481
Sumit K, Dipti S, Premanand Y, Mahendra Y (2013) Experimental and quantum chemical studies on corrosion inhibition effect of synthesized organic compounds on N80 steel in hydrochloric acid. Ind Eng Chem Res 52:14019–14029
Bataillon C, Brunet S (1994) Electrochemical impedance spectroscopy on oxide films formed on zircaloy in high temperature water. Electrochim Acta 39:455–465
Pradeep Kumar CB, Mohana KN (2014) Corrosion inhibition efficiency and adsorption characteristics of some Schiff bases at mild steel/hydrochloric acid interface. J Taiwan Inst Chem Eng 45:1031–1042
Kairi NI, Kassim J (2013) The effect of temperature on the corrosion inhibition of mild steel in 1 M HCl solution by Curcuma longa extract. Int J Electrochem Sci 8:7138–7155
Faustin M, Maciuk A, Salvin P, Roos C, Lebrini M (2015) Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: electrochemical and phytochemical studies. Corros Sci 92:287–300
Rajeswari V, Kesavan D, Gopiraman M, Viswanathamurthi P (2013) Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—inhibition of cast iron corrosion. Carbohydr Polym 95:288–294
Li Xianghong, Deng Shuduan, Hui Fu, Xie Xiaoguang (2014) Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corros Sci 78:29–42
Mohamed AAS, Hadeal hamdy MF (2013) Formulation and evaluation of betamethasone sodium phosphate loaded nanoparticles for ophthalmic delivery. J Clin Exp Ophthalmol 4:2
Dholakia M, Thakkar V, Patel N, Gandhi T (2012) Development and characterisation of thermo reversible mucoadhesive moxifloxacin hydrochloride in situ ophthalmic gel. J Pharm Bioallied Sci 4:42–45
Soayeda AA, Refaat HM, Noor El-Din DA (2013) Metal complexes of moxifloxacin–imidazole mixed ligands: characterization and biological studies. Inorg Chim Acta 406:230–240
Ma H, Chen S, Yin B, Zhao S, Liu X (2003) Impedance spectroscopic study of corrosion inhibition of copper by surfactants in the acidic solutions. Corros Sci 45:867–882
Solmaz R, Mert ME, Kardas G, Yazici B, Erbil M (2008) Adsorption and corrosion inhibition effect of 1,1′-thiocarbonyldiimidazole on mild steel in H2SO4 solution and synergistic effect of iodide ion. Acta Phys Chem Sin 24:1185–1191
Feng Y, Siow KS, Teo WK, Hsieh AK (1999) The synergistic effects of propargyl alcohol and potassium iodide on the inhibition of mild steel in 0.5 M sulfuric acid solution. Corros Sci 41:829–852
Obot IB, Obi-Egbedi NO, Umoren SA (2009) The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloric acid corrosion of aluminium. Corros Sci 51:276–282
Umoren SA, Li Y, Wang FH (2010) Synergistic effect of iodide ion and polyacrylic acid on corrosion inhibition of iron in H2SO4 investigated by electrochemical techniques. Corros Sci 52:2422–2429
Umoren SA, Ogbobe O, Igwe IO, Ebenso EE (2008) Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci 50:1998–2006
Pavithra MK, Venkatesha TV, Vathsala K, Nayana KO (2010) Synergistic effect of halide ions on improving corrosion inhibition behaviour of benzisothiozole-3-piperizine hydrochloride on mild steel in 0.5 M H2SO4 medium. Corros Sci 52:3811–3819
Ai JZ, Guo XP, Qu JE, Chen ZY, Zheng JS (2006) Adsorption behavior and synergistic mechanism of a cationic inhibitor and KI on the galvanic electrode. Colloids Surf A Physicochem Eng Aspects 281:147–155
Solmaz R, Kardas G, Culha M, Yazici B, Erbil M (2008) Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim Acta 53:5941
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nathiya, R.S., Perumal, S., Murugesan, V. et al. Expired Drugs: Environmentally Safe Inhibitors for Aluminium Corrosion in 1 M H2SO4 . J Bio Tribo Corros 4, 4 (2018). https://doi.org/10.1007/s40735-017-0120-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-017-0120-1