Abstract
Background
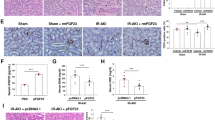
Prolonged cold ischemia time, the period from the start of perfusion with cold preservation fluid after cessation of circulation due to arterial clamping until transplantation in the recipient, could induce epithelial-to-mesenchymal transition (EMT) in renal tubular cells, a process associated with chronic graft damage. In this context, everolimus (EVE) and sulodexide (SUL) could potentially slow down this process.
Methods
To assess whether SUL (50 μg/ml), EVE (at 5, 10, 100 nM) or their combination were able to inhibit EMT in human renal epithelial proximal tubular cells (HK-2) reoxygenated after 24 h under hypoxic conditions, we used classical biomolecular strategies.
Results
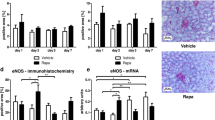
Hypoxia induced upregulation of alpha smooth muscle actin (α-SMA), fibronectin (FN) and vimentin at gene-expression and α-SMA and FN at protein levels. However, the addition, after reoxygenation, of SUL plus low-dose EVE (5 nM) to the cell culture reversed this condition. Moreover, SUL and EVE were able to inhibit the hypoxia-induced Akt phosphorylation in HK2 cells and their morphological changes. Similarly, SUL was able to reverse the hyper-expression of EMT markers induced by high EVE dosage (100 nM) in cells cultured under both normoxic and hypoxic conditions.
Conclusions
Our data reveal, for the first time, that sulodexide, alone or combined to low doses of everolimus, may hinder EMT in renal cells following hypoxia or minimize fibrotic complications due to high dosage of mammalian target of rapamycin inhibitors.









Similar content being viewed by others
References
Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T (2012) New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 8(6):339–347
Kwiatkowski A, Wszoła M, Kosieradzki M, Danielewicz R, Ostrowski K, Domagala P, Lisik W, Fesołowicz S, Michalak G, Trzebicki J, Durlik M, Paczek L, Rowiński W, Chmura A (2009) The early and long term function and survival of kidney allografts stored before transplantation by hypothermic pulsatile perfusion. A prospective randomized study. Ann Transplant 14:14–17
Moers C, Pirenne J, Paul A, Ploeg RJ, Machine Preservation Trial Study Group (2012) Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 366(8):770–771
Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML (2006) Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant 6:1111–1131
Meier-Kriesche HU, Schold JD, Srinivas TR, Kapplan B (2004) Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4:378–383
US Renal Data System, USRDS (2013) Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013
Ojo AO (2006) Cardiovascular complications after renal transplantation and their prevention. Transplantation 82:603–611
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349:2326–2333
Chapman JR, O’Connell PJ, Nankivell BJ (2005) Chronic renal allograft dysfunction. J Am Soc Nephrol 16(10):3015–3026
Paul LC (1999) Chronic allograft nephropathy: an update. Kidney Int 56:783–793
Wynn TA (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 117:524–529
Carew RM, Wang B, Kantharidis P (2012) The role of EMT in renal fibrosis. Cell Tissue Res 347:103–116
Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF (2005) Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant 5:1367–1374
Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC (2006) Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 6:2937–2946
Opelz G, Döhler B (2007) Multicenter analysis of kidney preservation. Transplantation 83:247
Hernández D, Estupiñán S, Pérez G, Rufino M, González-Posada JM, Luis D, Delgado P, Rodríguez A, Marrero D, Porrini E, Torres A (2008) Impact of cold ischemia time on renal allograft outcome using kidneys from young donors. Transplant Int 21(10):955–962
Salahudeen AK, Haider N, May W (2004) Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int 65(2):713–718
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2(2):1303–1353
Pontrelli P, Rossini M, Infante B et al (2008) Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation 85:125–134
Geissler EK, Schlitt HJ (2010) The potential benefits of rapamycin on renal function, tolerance, fibrosis, and malignancy following transplantation. Kidney Int 78(11):1075–1079
Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, Liu F (2012) Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7(3):e33626
Masola V, Onisto M, Zaza G, Lupo A, Gambaro G (2012) A new mechanism of action of sulodexide in diabetic nephropathy: inhibits heparanase-1 and prevents FGF-2-induced renal epithelial-mesenchymal transition. J Transl Med 10:213
Masola V, Zaza G, Gambaro G (2014) Sulodexide and glycosaminoglycans in the progression of renal disease. Nephrol Dial Transplant 29(Suppl 1):i74–i79
Masola V, Zaza G, Granata S, Gambaro G, Onisto M, Lupo A (2013) Everolimus-induced epithelial to mesenchymal transition in immortalized human renal proximal tubular epithelial cells: key role of heparanase. J Transl Med 11:292
Kosieradzki M, Rowiński W (2008) Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc 40(10):3279–3288
van der Vliet JA, Warlé MC (2013) The need to reduce cold ischemia time in kidney transplantation. Curr Opin Organ Transplant 18(2):174–178
Ponticelli C (2014) Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant 29(6):1134–1140
Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17(6):1503–1520
Perico N, Cattaneo D, Sayegh MH, Remuzzi G (2004) Delayed graft function in kidney transplantation. Lancet 364(9447):1814–1827
Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M (2004) Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 65:871–880
Gottmann U, Mueller-Falcke A, Schnuelle P, Birck R, Nickeleit V, van der Woude FJ, Yard BA, Braun C (2007) Influence of hypersulfated and low molecular weight heparins on ischemia/reperfusion: injury and allograft rejection in rat kidneys. Transpl Int 20(6):542–549
Gedik HS, Korkmaz K, Erdem H, Karakilic E, Lafci G, Ankarali H (2012) Protective effect of heparin in the end organ ischemia/reperfusion injury of the lungs and heart. J Cardiothorac Surg 7:123
Zhou T, Chen JL, Song W, Wang F, Zhang MJ, Ni PH, Geng JG (2002) Effect of N-desulfated heparin on hepatic/renal ischemia reperfusion injury in rats. World J Gastroenterol 8(5):897–900
Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF, Sirolimus CONVERT Trial Study Group, Sirolimus CONVERT Trial Study Group (2009) Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 87:233
Holdaas H, Bentdal Ø, Pfeffer P, Mjørnstedt L, Solbu D, Midtvedt K (2008) Early, abrupt conversion of de novo renal transplant patients from cyclosporine to everolimus: results of a pilot study. Clin Transplant 22:366
Budde K, Becker T, Sommerer C et al (2009) Analysis of renal function in everolimus/enteric-coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the Zeus study. Am J Transplant 9:259 (Abstract 237)
Weir M, Mulgaonkar S, Pearson T et al (2009) Mycophenolatemofetil/sirolimus maintenance therapy after calcineurin inhibitor withdrawal in renal transplant recipients: 2-year outcomes of the Spare-the-Nephron (STN) trial. Am J Transplant 9:200 (Abstract 33)
Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, Heng AE, Onno C, Buchler M, Girardot-Seguin S, Hurault de Ligny B (2009) Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 9:1115
Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F, ZEUS Study Investigators (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377(9768):837–847
Hong JC, Kahan BD (2001) A calcineurin antagonist-free induction strategy for immunosuppression in cadaveric kidney transplant recipients at risk for delayed graft function. Transplantation 71(9):1320–1328
Pallet N, Legendre C (2013) Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf 12(2):177–186
Lieberthal W, Fuhro R, Andry CC (2001) Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 281(4):F693–F706
Campistol JM, Cockwell P, Diekmann F, Donati D, Guirado L, Herlenius G, Mousa D, Pratschke J, San Millán JC (2009) Practical recommendations for the early use of m-TOR inhibitors (sirolimus) in renal transplantation. Transpl Int 22:681–687
Dantal J, Berthoux F, Moal MC (2010) Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-months results of a randomized, multicenter trial. Transpl Int 23(11):1084–1093
Zaza G, Tomei P, Ria P, Granata S, Boschiero L, Lupo A (2013) Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin Dev Immunol 2013:403280
Zaza G, Granata S, Tomei P, Masola V, Gambaro G, Lupo A (2014) mTOR inhibitors and renal allograft: Yin and Yang. J Nephrol 5:495
Breuleux M, Klopfenstein M, Stephan C, Doughty CA, Barys L, Maira SM, Kwiatkowski D, Lane HA (2009) Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther 8(4):742–753
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26(13):1932–1940
Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12(4):487–502
Slomovitz BM, Coleman RL (2012) The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res 18(21):5856–5864
Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM (2003) Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 63(10):2658–2664
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaza, G., Masola, V., Granata, S. et al. Sulodexide alone or in combination with low doses of everolimus inhibits the hypoxia-mediated epithelial to mesenchymal transition in human renal proximal tubular cells. J Nephrol 28, 431–440 (2015). https://doi.org/10.1007/s40620-015-0216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0216-y