Abstract
Purpose
The serum metabolic changes occurring during the transition from hypothyroidism to euthyroidism are not known. This study aimed to determine the metabolomic profile in hypothyroid patients before (HypoT0) and after (HypoT1) euthyroidism achieved through levothyroxine (L-T4) treatment.
Methods
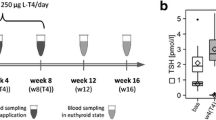
Eighteen patients with overt primary hypothyroidism were recruited for the study. All patients were treated with L-T4 to achieve euthyroidism. Thyrotropin (TSH), free thyroxine (FT4), free triiodothyronine (FT3) and metabolomics profiles were measured before and after 3 months of treatment. The euthyroid control group consisted of 28 healthy volunteers. Metabolomics analysis was performed using Nuclear Magnetic Resonance (NMR) spectroscopy.
Results
1H NMR-based metabolomics profiling of patients with newly diagnosed hypothyroidism (HypoT0) showed significantly higher levels of citrate, creatinine, glycerol, myo-inositol and serine, and lower levels of proline and taurine compared to controls. Interestingly, some metabolic changes were persistent three months after pharmacological treatments, despite normal serum TSH and thyroid hormone concentrations (HypoT1). When an Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) model was built to evaluate possible differences in the metabolic profile between HypoT0 and HypoT1, the data obtained were not significantly different.
Conclusion
These results suggest that metabolic changes in the patients with hypothyroidism may persist after normalization of serum levels of FT3, FT4, and TSH, which currently represent the gold standard in laboratory testing for diagnosis and evaluation of thyroid pathology. So, the metabolomics approach may contribute to integrate classical hormone assays and to determine the euthyroid status achievement with greater efficacy.






Similar content being viewed by others
References
Chakera AJ, Pearce SHS, Vaidya B (2012) Treatment for primary hypothyroidism: current approaches and future possibilities. Drug Des Devel Ther 6:1–11
Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94(2):355–382
Iwen KA, Schröder E, Brabant G (2013) Thyroid hormones and the metabolic syndrome. Eur Thyroid J 2:83–92
Brent GA (2012) Hypothyroidism and thyroiditis. In: Melmed SP, Larsen PR, Kronenberg HM (eds) Williams Textbook of Endocrinology. Elsevier, Philadelphia
Comte B, Vidal H, LavilleM RJP (1990) Influence of thyroid hormones on gluconeogenesis from glycerol in rat hepatocytes: a dose-response study. Metabolism 39(3):259–263
Feng X, Jiang Y, Meltzer P, Yen PM (2000) Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol Baltim Md 14(7):947–955
Torrance CJ, Devente JE, Jones JP, Dohm GL (1997) Effects of thyroid hormone on GLUT4 glucose transporter gene expression and NIDDM in rats. Endocrinology 138(3):1204–1214
Duntas LH (2002) Thyroid disease and lipids. Thyroid Off J Am Thyroid Assoc 12(4):287–293
Krotkiewski M (2002) Thyroid hormones in the pathogenesis and treatment of obesity. Eur J Pharmacol 440(2–3):85–98
Zucchi R (2020) Thyroid Hormone Analogues: An Update. Thyroid. https://doi.org/10.1089/thy.2020.0071
Joharapurak AA, Dhote VV, Jain MR (2012) Selective thyromimetics using receptor and tissue selectivity approaches: prospects for dyslipidemia. J Med Chem 55(12):5649–5675
Mondal S, Mugesh G (2017) Novel thyroid hormone analogues, enzyme inhibitors and mimetics, and their action. Mol Cell Endocrinol 458:91–104
Brent GA (1994) The molecular basis of thyroid hormone action. N Engl J Med 331(13):847–853
Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A (2001) Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEB J 15(6):1006–1013
Tancevski I, Rudling M, Eller P (2011) Thyromimetics: a journey from bench to bed-side. Pharmacol Ther 131(1):33–39
Riis ALD, Jørgensen JOL, Gjedde S, Nørrelund H, Jurik AG, Nair KS, Irvasen P, Weeke J, Møller N (2005) Whole body and forearm substrate metabolism in hyperthyroidism: evidence of increased basal muscle protein breakdown. Am J Physiol Endocrinol Metab 288(6):E1067–1073
Brennan MD, Coenen-Schimke JM, Bigelow ML, Nair KS (2006) Changes in skeletal muscle protein metabolism and myosin heavy chain isoform messenger ribonucleic acid abundance after treatment of hyperthyroidism. J Clin Endocrinol Metab 91(11):4650–4656
Nørrelund H, Hove KY, Brems-Dalgaard E, Jurik AG, Nielsen LP, Nielsen S, Jørgensen JO, Weeke J, Møller N (1999) Muscle mass and function in thyrotoxic patients before and during medical treatment. Clin Endocrinol (Oxf) 51(6):693–699
Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS (2006) The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid Off J Am Thyroid Assoc 16(4):375–380
Yen PM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81(3):1097–1142
Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP (2013) The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 98(7):2936–2943
Brown SJ, Bremner AP, Hadlow NC, Feddema P, Leedman PJ, O’Leary PC, Walsh JP (2016) The log TSH-free T4 relationship in a community-based cohort is nonlinear and is influenced by age, smoking and thyroid peroxidase antibody status. Clin Endocrinol (Oxf) 85(5):789–796
Deidda M, Piras C, Dessalvi CC, LocciE BL, Torri F, Ascedu F, Atzori L, Mercuro G (2015) Metabolomic approach to profile functional and metabolic changes in heart failure. J Transl Med 13:297
Wishart DS (2019) Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol Rev 99(4):1819–1875
Piras C, Arisci N, Poddighe S, Liggi S, Mariotti S, Atzori L (2017) Metabolomic profile in hyperthyroid patients before and after antithyroid drug treatment: Correlation with thyroid hormone and TSH concentration. Int J Biochem Cell Biol 93:119–128
Deidda M, Piras C, Binaghi G, Congia D, Pani A, Boi A, Sanna F, Rossi A, Loi B, CadedduDessalvi C, Atzori L, Porcu M, Mercuro G (2019) Metabolomic fingerprint of coronary blood in STEMI patients depends on the ischemic time and inflammatory state. Sci Rep 9(1):312
Lefebvre B, Sasaki R, Golotvin S, Nicholls AW (2010b) Intelligent Bucketing for Metabonomics −Part 2. (20 Jan 2010). https://www.acdlabs.co.uk/download/ publ/2004/intelbucket2. pdf/
van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ (2006) Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genom 7:142
Bro R, Smilde AK (2014) Principal component analysis. Anal Methods 6:2812–2831
Bylesjö M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J (2006) OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J Chemom 20:341–351
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 78(13):4430–4442
Aickin M, Gensler H (1996) Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 86(5):726–728
Chou KM, Chiu SYH, Chen CH, Yang NI, Huang BY, Sun CY (2011) Correlation of clinical changes with regard to thyroxine replacement therapy in hypothyroid patients: focusing on the change of renal function. Kidney Blood Press Res 34(5):365–372
Fallahi P, Ferrari SM, Elia G, Ragusa F, Paparo SR, Caruso C, Guglielmi G, Antonelli A (2018) Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev Endocr Metab Disord 19(4):349–354
Kutateladze TG (2010) Translation of the phosphoinositide code by PI effectors. Nat Chem Biol 6(7):507–513
Downes CP, Macphee CH (1990) myo-inositol metabolites as cellular signals. Eur J Biochem 193(1):1–18
Ohye H, Sugawara M (2010) Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood) 235(4):424–433
Grasberger H, Van Sande J, Hag-DahoodMahameed A, Tenenbaum-Rakover Y, Refetoff S (2007) A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab 92(7):2816–2820
Xue LL, Chen HH, Jiang JG (2017) Implications of glycerol metabolism for lipid production. Progress Lipid Res 68:12–25
Lee YP, Lardy HA (1965) Influence of thyroid hormones on L-alpha-glycerophosphate dehydrogenases and other dehydrogenases in various organs of the rat. J Biol Chem 240:1427–1436
Okamura K, Taurog A, Krulich L (1981) Hypothyroidism in severely iodine-deficient rats. Endocrinology 109(2):464–468
Dümmler K, Müller S, Seitz HJ (1996) Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem J 317:913–918
Krämer R, Palmieri F (1992) Chapter 16 Metabolite carriers in mitochondria. In: Ernster L (ed) New Comprehensive Biochemistry. Elsevier, 23:359–384
Giudetti AM, Leo M, Siculella L, Gnoni GV (2006) Hypothyroidism down-regulates mitochondrial citrate carrier activity and expression in rat liver. Biochim Biophys Acta 1761(4):484–491
Werner SC, Ingbar SH, Braverman LE, Utiger RD (2005) Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text. Lippincott Williams & Wilkins 5th ed
Verhelst J, Berwaerts J, Marescau B, Abs R, Neels H, Mahler C, Deyn PPD (1997) Serum creatine, creatinine, and other guanidino compounds in patients with thyroid dysfunction. Metabolism 4(9):1063–1067
Verpoorte R, Choi YH, Kim HK (2007) NMR-based metabolomics at work in phytochemistry. Phytochem Rev 6:3–14
Dirican M, Taş S, Sarandöl E (2007) High-dose taurine supplementation increases serum paraoxonase and arylesterase activities in experimental hypothyroidism. Clin Exp Pharmacol Physiol 34(9):833–837
Wu S, Tan G, Dong X, Zhu Z, Li W, Lou Z, Chai Y (2013) Metabolic profiling provides a system understanding of hypothyroidism in rats and its application. PLoS ONE 8(2):e55599
McGrowder DA, Fraser YP, Gordon L, Crawford TV, Rawlins JM (2011) Serum creatine kinase and lactate dehydrogenase activities in patients with thyroid disorders. Niger J Clin Pract 14(4):454–459
Strasberg GD (1983) Lactate dehydrogenase isozyme 1. Arch Intern Med 143(10):2023
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
LA and SM conceived the study and supervised the project. NA obtained the samples and clinical details. CP, AB. VPL and LT performed metabolomics experiments and data analysis. CP, MP, and NA wrote the first draft of the manuscript, and CP, MP, VPL, LA, SM, and NA, contributed to the final version. CP, MP, LA, and SM critically reviewed the data and the manuscript. All the authors have read and approved the whole content of this submitted manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
This study has been performed according to the Declaration of Helsinki and was approved by the Ethical Committee of the University Hospital of Cagliari.
Informed consent
All the participants were provided written informed consent to participate and to publish data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piras, C., Pibiri, M., Leoni, V.P. et al. Analysis of metabolomics profile in hypothyroid patients before and after thyroid hormone replacement. J Endocrinol Invest 44, 1309–1319 (2021). https://doi.org/10.1007/s40618-020-01434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01434-y