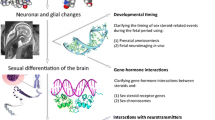
Abstract
Purpose of Review
This review discusses the current state of knowledge on sex differences in the epigenetic regulation in the brain and highlights its relevance for the environmental studies of brain and behavior.
Recent Findings
Recent evidence shows that epigenetic mechanisms are involved in the control of brain sexual differentiation and in memory-enhancing effects of estradiol in females. In addition, several studies have implicated epigenetic dysregulation as an underlying mechanism for sex-specific neurobehavioral effects of environmental exposures.
Summary
The area of sex-specific neurepigenetics has a great potential to improve our understanding of brain function in health and disease. Future neuropigenetic studies will require the inclusion of males and females and would ideally account for the fluctuating hormonal status in females which is likely to affect the epigenome. The implementation of cutting-edge methods that include epigenomic characterization of specific cell types using latest next-generation sequencing approaches will further advance the area.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–83.
McEwen BS. Introduction: the end of sex as we once knew it. Physiol Behav. 2009;97(2):143–5.
Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 2016;18(4):373–83.
McCarthy MM. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci. 2016;18(4):361–72.
Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33(1):3–17.
Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9(11):e1001349.
Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–39.
Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34(6):1054–63.
Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80.
Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31(3):363–73.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902.
Bisson JI, Cosgrove S, Lewis C, Robert NP. Post-traumatic stress disorder. BMJ. 2015;351:h6161.
Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neurosci : Rev J Neurobiol Neurol Psychiatry. 2016;22(5):447–63.
Kundakovic M, Jaric I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes. 2017;8(3):104.
McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, et al. The epigenetics of sex differences in the brain. J Neurosci : Off J Soc Neurosci. 2009;29(41):12815–23.
Menger Y, Bettscheider M, Murgatroyd C, Spengler D. Sex differences in brain epigenetics. Epigenomics. 2010;2(6):807–21.
Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110(24):9956–61.
•• Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci U S A. 2015;112(22):6807–13. This study shows that prenatal exposure to a widely used plasticizer, bisphenol A, is associated with sex-specific epigenetic changes in rodents and humans. In rodents, prenatally induced epigenetic alterations are linked to changes in brain gene expression and behavior in the adulthood
Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Frontiers in psychiatry. 2013;4:78.
Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40(1):141–53.
Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97.
Verdin E, Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16(4):258–64.
Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–57.
Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8.
Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95.
Magnani L, Lupien M. Chromatin and epigenetic determinants of estrogen receptor alpha (ESR1) signaling. Mol Cell Endocrinol. 2014;382(1):633–41.
Lappalainen T, Greally JM. Associating cellular epigenetic models with human phenotypes. Nat Rev Genet. 2017;18(7):441–51.
Arnold AP, McCarthy MM. Sexual differentiation of the brain and behavior: a primer. In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st century: from basic to clinical. New York, NY: Springer New York; 2016. p. 2139–68.
Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–6.
Puts D, Motta-Mena NV. Is human brain masculinization estrogen receptor-mediated? Reply to Luoto and Rantala. Horm Behav. 2018;97:3–4.
Nishino K, Hattori N, Tanaka S, Shiota K. DNA methylation-mediated control of Sry gene expression in mouse gonadal development. J Biol Chem. 2004;279(21):22306–13.
Kuroki S, Matoba S, Akiyoshi M, Matsumura Y, Miyachi H, Mise N, et al. Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science. 2013;341(6150):1106–9.
•• Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–7. This study provides evidence that epigenetic mechanisms regulate sexual differentiation of the brain and behavior. It also opens a new possibility that brain feminization is an active process requiring DNA methylation-mediated suppression of brain masculinization
Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150(9):4241–7.
Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152(7):2760–7.
Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, et al. Epigenetic control of female puberty. Nat Neurosci. 2013;16(3):281–9.
Sundstrom Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front Neurosci. 2014;8:380.
Yonkers KA, O'Brien PS, Eriksson E. Premenstrual syndrome. Lancet. 2008;371(9619):1200–10.
Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28(Suppl 3):1–23.
Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017;95(1–2):539–62.
Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–9.
Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12(7):2549–54.
Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703(1–2):26–30.
Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–801.
Duclot F, Kabbaj M. The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biol. 2015;16(1):256.
•• Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010;107(12):5605–10. This study shows that the epigenomes of adult hippocampal cells are responsive to estrogen and that epigenetic mechanisms are involved in memory-enhancing effects of estradiol
Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32(7):2344–51.
Jaric I, Rocks D, Greally JM, Suzuki M, Kundakovic M. Dynamic changes in neuronal chromatin organization across the estrous cycle are linked to anxiety-related phenotypes. Program No. 74.09. 2017 Neuroscience Meeting Planner. Washington, DC; Society for Neuroscience, 2017. Online.
Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120(8):1190–4.
Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–52.
Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18(21):4046–53.
Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65.
Kundakovic M. In utero bisphenol a exposure and epigenetic programming of neurobehavioral outcomes. In: Hollar D, editors. Epigenetics, the environment, and children’s health across lifespans: Springer; 2016. p. 67–92.
Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–62.
Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–93.
Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28(1):111–8.
Tetel MJ, Pfaff DW. Contributions of estrogen receptor-alpha and estrogen receptor-ss to the regulation of behavior. Biochim Biophys Acta. 2010;1800(10):1084–9.
Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012;17(6):584–96.
Blaze J, Scheuing L, Roth TL. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev Neurosci. 2013;35(4):306–16.
Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10(5):408–17.
Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35(50):16362–76.
Kundakovic M, Jiang Y, Kavanagh DH, Dincer A, Brown L, Pothula V, et al. Practical guidelines for high-resolution epigenomic profiling of nucleosomal histones in postmortem human brain tissue. Biol Psychiatry. 2017;81(2):162–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marija Kundakovic declares that there are no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Synthetic Chemicals and Health
Rights and permissions
About this article
Cite this article
Kundakovic, M. Sex-Specific Epigenetics: Implications for Environmental Studies of Brain and Behavior. Curr Envir Health Rpt 4, 385–391 (2017). https://doi.org/10.1007/s40572-017-0172-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-017-0172-x