Abstract
Purpose of Review
Socioeconomic status (SES) has long been understood to be a key determinant of the distribution of tuberculosis (TB), and the role of social factors has long been a truism of TB epidemiology. We review studies that have examined the social determinants of TB in the USA in the past 20 years. We pay particular attention to how the findings of these studies fit within the framework of fundamental cause theory and argue that a more explicit linkage with fundamental cause theory is critical for understanding the current state of TB health disparities in the USA and for charting a way towards TB elimination in the USA.
Recent Findings and Summary
Our review finds that while in the past 20 years there have been studies that have documented the ongoing association between social factors and TB disease in the USA, few studies explore the precise mechanisms through which social factors continue to influence TB patterns. We advocate for a move towards a system-based approach both in theory development and analyses, allowing for the incorporation of more complex social dynamics to address long-standing disparities in TB disease.
Similar content being viewed by others
Introduction
Social factors have been the key to determining patterns of tuberculosis (TB) since we began recognizing the disease. Indeed, even before Robert Koch isolated Mycobacterium tuberculosis (MTB), the intense social stigma associated with TB disease was a reflection of its associations with poverty [1]. This understanding is most memorably reflected in Dubos’ description of MTB as a “necessary, but not sufficient” component of the cycle of TB that needs to be accompanied by the suite of social and environmental factors that facilitate infection as well as progression to active disease [1]. The decline of TB in the USA is often cited as a key feature of the epidemiologic transition, in which infectious diseases were replaced by chronic illnesses as the primary causes of mortality [2]. However, the improvements in living standards that led to the decline of TB in the USA during the early twentieth century were not distributed equally across the population [2]. For example, as TB incidence fell, racial disparities in TB infection in rapidly expanding Northern cities, such as Chicago, New York, and Baltimore grew [3, 4]. As TB incidence, prevalence, and mortality in the USA have continued to fall, the inverse relationship between socioeconomic status and TB remains, but its contours have changed.
As an airborne, infectious disease, anyone can be exposed to MTB and thus be at risk for subsequent development of the disease. However, as we have come closer to the goal of domestic TB elimination, the disease has become concentrated in, although by no means exclusively so, in poorer, socially marginalized populations. In 2017, the rate among foreign-born persons was 15 times that of US-born persons [5]. And while an estimated 70% of TB cases occur among foreign-born persons, those among US-born individuals are disproportionately likely to be among those living in poverty [5].
Despite the clear role SES plays in structuring the risk factors associated with TB infection and disease, there has been little, if any, discussion of TB—or any other infectious disease—within the framework of the fundamental social causes of health and health disparities outlined by Link and Phelan [6]. Briefly, fundamental cause theory (FCT) states that as the ability to control a particular disease increases, socioeconomic disparities in that disease are likely to grow. This is because the wealthiest and more-advantaged individuals will have preferential access to treatment and prevention, saddling the least well-off with the residual burden of disease. The present moment—when the rate of decline in US TB rates has slowed [7]—is an important one in which to revisit our understanding of the relationship between SES and the risk of TB infection and disease in the USA, if we are to make progress towards the goal of domestic TB elimination.
In 1989, the CDC defined the goal of domestic TB elimination as reaching an incidence rate of less than 1 case per 1 million individuals by 2010, echoed by the World Health Organization (WHO) in their framework for addressing the global TB epidemic [8••]. As of 2017, however, the TB incidence rate in the USA was still 28 times that of the elimination standard [9]. While those working in TB control and research have long known that the social environment is a critical determinant of the epidemiology of TB in the USA, the focus of research has remained largely biomedical.
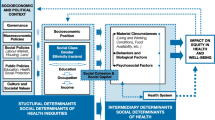
Our goal for this review is to provide an updated understanding of the mechanisms linking SES to TB disparities. To do this, we will review studies that have examined the social determinants of TB in the USA in the past 20 years. We pay particular attention to how the findings of these studies fit within the framework of FCT and argue that a more explicit linkage with FCT is critical for understanding the current state of TB disparities in the USA and for charting a path to domestic TB elimination. Our review is organized by individual social factors that have received the most explicit attention. However, all of these factors are components of a larger social environment; as such, their effects cannot easily be disentangled. Indeed, these social factors are best understood as part of a constellation of factors patterning the distribution of TB in the USA. We conceptualize the relationship between SES and TB as operating through two domains: distal mechanisms (e.g., race and nativity status) that structure exposure to more proximal mechanisms (e.g., physical environment and access to health care) (Fig. 1). Additionally, we propose that these social factors may exert their influence at multiple points in the natural history of TB disease. We conclude this review by offering next steps to consider for those at all levels of TB control: researchers, practitioners, and policy-makers.
Current Studies Examining Social Factors and TB in the USA
Socioeconomic Status
Socioeconomic status (SES), encompassing income, education, wealth, and other factors is a critical predictor of risk for a wide array of infectious and non-communicable diseases. Deemed a fundamental cause of both disease and health disparities, FCT posits that the relationship between SES and health is overdetermined, and that even when one proximal disease risk is blocked, many other pathways remain [6]. In the case of TB, SES operates through a number of pathways that impact both the prevalence and sociodemographic distribution of TB disease. These pathways include, but are not limited to, the physical environment, nutrition, access to health care, and exposure to psychosocial stress. For example, the physical environment may influence rates of TB exposure, as well as access to health care and visibility to public health surveillance. In addition, a primary mechanism linking SES to subsequent TB disease is immune function: Malnutrition, diabetes, and comorbidities such as HIV infection (all conditions resulting in diminished immune function) likely increase susceptibility to developing active TB disease following latent TB infection (LTBI), and all of these are known to be linked to individual and household SES [10,11,12].
Recent studies report associations between low SES and the overall prevalence of TB disease [5], LTBI alone [13], and of recent TB infection [14, 15]. This work has been extended to include the risk of development of multi-drug resistant (MDR)-TB [16]. Yet, while we know that SES has long been and continues to be a major determinant of TB in the USA, studies that explicitly examine the mechanisms through which SES is related to TB risk in the present day on TB are virtually nonexistent.
Nativity Status
In 2017, 70% of TB cases in the USA occurred among foreign-born persons, an incidence rate 15 times that of US-born persons [5]. This reflects a complex relationship between global inequality, domestic SES, and nativity: TB risk among individuals who have migrated to the USA is influenced by the relative TB burden in their countries of origin in addition to their SES in the USA [17•, 18]. Consequently, in the USA, nativity status is related to the distribution of TB in two main ways: One, given that much of the TB in the USA results from reactivation of LTBI among immigrants infected in their countries of origin, the prevalence of TB is directly related to the global burden of TB; and two, nativity status may in some instances be associated with exposure to risks related to low SES after arriving in the USA, impacting both the risk of acquiring TB infection after arrival and progression to disease.
Thus, when examining the role of nativity status in shaping the distribution of TB, it is necessary to classify cases as resulting from reactivation of LTBI separately from those resulting from recent transmission of MTB, though the tools for such classification are inherently limited. For example, a recent study in Michigan estimated that more than half of TB cases among US-born individuals resulted from recent transmission compared to 22% of foreign-born cases [14].
Screening and treatment policies for TB in the USA are characterized by gaps at the federal and state level that may contribute to missing otherwise preventable TB cases in foreign-born populations [19]. Screening for active TB is only required for those individuals wishing to establish permanent residency in the USA [19]. Thus, for the large numbers of individuals who migrate to the USA on temporary visas (i.e., work or student visas) or through other means, screening and treatment for TB is more fragmented. In one study of foreign-born persons diagnosed with TB, 13% had temporary visas and 25% had no visa [20]. This may result in fewer opportunities for screening and reduced follow-through for LTBI screening especially [20, 21].
Studies examining nativity disparities in the distribution of TB also report significant differences by race/ethnicity, nativity, and SES [22]. Indeed, the extent to which SES predicts TB risk is related to nativity. For example, national TB incidence data suggest that SES is a poorer predictor of TB for foreign-born populations compared to US-born populations. Among US-born populations, there is a steep SES gradient observable in TB incidence whereby lower SES is associated with increased prevalence of TB [23]. This gradient is much less steep for foreign-born populations [24], highlighting that SES may operate differentially for foreign-born and US-born populations. Thus, analyses stratified by nativity status are often necessary to tease apart the differential effects of the social environment.
Much of the recent work on nativity disparities in the USA has focused on the experience of immigrants, particularly Latino immigrants. The stigma associated with TB disease can be amplified among foreign-born populations [25], especially during times of hostility to immigration [26]. Several studies have reported that foreign-born persons with TB experience high levels of social isolation and perceptions of stigma [25, 27]. In addition, this population also experiences reduced adherence to TB drug treatment resulting in worse TB outcomes, including increased likelihood of the development of drug resistance [28].
A study of TB incidence in correctional facilities found that nearly a third of all the foreign-born Latino individuals with TB were in a facility category that includes Immigration and Customs Enforcement Detention Centers [29] in which crowded conditions may facilitate transmission. Additionally, the psychological duress of incarceration likely diminishes the immune response, increasing the likelihood of progressing to active disease if infected. The above study also found that both foreign-born birth and the recentness of arrival in the USA were predictors of whether a Latino individual was diagnosed with TB while incarcerated. This suggests that the current level of hostility to immigration in the US could result in an increased risk of TB among targeted immigrant populations.
Race/Ethnicity
One of the key impediments to a robust understanding of racial/ethnic disparities related to TB is the biologizing of race that has persisted in the literature on racial disparities in TB risk. Consistent with results on other health disparities, there are entrenched racial/ethnic disparities in the distribution of TB in the USA. However, in contrast to the large majority of other recent disparities research, these differences are frequently attributed to biological differences in susceptibility to TB infection. Much of this work is predicated on flawed research [30] that has implied that Blacks have increased genetic susceptibility to MTB infection [31, 32] and progression to TB disease [33]. Given the decades of research showing greater heterogeneity in susceptibility within racial groups rather than across racial groups [34], it is unlikely that genetic differences account for disparities observed in the acquisition and progression of TB. More likely, group-level differences in susceptibility to TB infection or disease are linked to SES factors, such as residential segregation [30].
Racial disparities are observable in the prevalence of TB disease, LTBI, and in the likelihood of recent transmission. Notably, US racial disparities in TB differ significantly by nativity status. At the national level, among foreign-born individuals, the highest TB case rate is among Asians, followed by non-Hispanic Blacks and Native Hawaiian/Pacific Islanders [5]. Among US-born individuals, non-Hispanic Blacks account for the largest portion of TB cases (37%), while the highest case rate is among Native Hawaiians and Pacific Islanders [5]. Racial disparities are also observable in the incidence of LTBI [35]. In both national and regional studies among the US-born, Blacks had a greater prevalence of LTBI than Whites [13, 36].
Racial disparities are also apparent when we stratify cases by whether they represent recent transmission or reactivation of LTBI: For example, using Michigan surveillance data, one study found that Blacks were at highest risk of recent infection, while Asians were at highest risk of TB disease resulting from reactivation of LTBI [22]. Another study using secondary contacts of active TB cases to infer transmission dynamics found that close contacts of African-American TB patients had twice the risk of infection compared to close contacts of White TB patients [24]. Moreover, a study examining both recent transmission as well as extensive recent transmission (a transmission cluster of at least six cases) found that extensive recent transmission occurred disproportionately among racial minorities and homeless individuals [37].
Physical Environment
In recent years, much of the research into the social determinants of TB has been focused on the impact of the physical environment, specifically neighborhoods, on the risk of TB infection and disease. From a transmission dynamics viewpoint, the size of the population that is susceptible to infection and how densely these persons are distributed in space are attributes of the neighborhood environment that directly impacts the rate of MTB transmission. However, it is important to understand these neighborhood attributes as products of a system of social stratification.
For example, racial residential segregation has been hypothesized to explain persistent racial/ethnic disparities in TB infection because it operates via multiple pathways: Segregation concentrates minorities and individuals of lower SES in communities characterized by overcrowded housing, high population density, and limited access to care [17•], in addition to increasing the rate of within- vs. between-group contact, thus potentially concentrating disease within segregated groups. In an examination of zipcode-level variation in TB risk in New Jersey during the resurgence of TB in the 1980s and 1990s, Acevedo-Garcia [38] found that indices of residential segregation were predictive of the presence of environmental and social risk factors for TB—as well as highly elevated TB risk—among immigrants, Blacks, and Hispanics. Several more recent studies of major urban environments report concentrated TB zones in areas of high neighborhood socioeconomic disadvantage [23, 39,40,41]. A series of studies in King County, Washington, showed that neighborhood-level socioeconomic status was associated with TB incidence, more severe TB disease, and genotypic clustering (a proxy for MTB transmission) [39, 42, 43]. Another study of genotypic clustering in Southern California reported case clusters defined primarily by race/ethnicity and country of origin [44]. This is consistent with other work in California [45] that found that census tracts with lower median income, more racial/ethnic minorities, and more immigrants had higher rates of pediatric TB (another method for inferring recent transmission).
Work on the physical environment may also hold keys to understanding racial disparities in TB. A Michigan study found that among the US-born, including markers of neighborhood disadvantage washed out the association between individual-level race and incidence of recent transmission in an analytical model [14]. Treating race as an independent variable ignores the macrosocial processes that systematically skew the distribution of individuals into disadvantaged environments as a function of race and class. Because of this, Acevedo-Garcia argues that residential segregation is one manifestation of racism that is a critical determinant of disparities in infectious disease between US minorities and Whites [30], echoing the argument by Williams and Collins that racial residential segregation is a fundamental cause of health disparities [46]. The contribution of residential segregation to racial disparities in TB likely reflects the enduring effects of migration patterns of the early 1900s in which living conditions for minorities and Whites diverged as populations increasingly moved to urban environments [3].
Directions for the Future
Challenges to Elimination
Despite significant progress towards domestic TB elimination, recent slowdowns in the rate of improvement have increased pessimism about near-term reachability of this goal. A recent transmission modeling analysis suggests that domestic TB elimination among US-born individuals is unlikely before 2100, far later than the 2010 US benchmark and 2035 WHO target for global elimination, and that elimination by even this time is unlikely among individuals born outside of but residing in the USA [47, 48•].
One explanation for the declining rate of improvement is that as incidence rates fall, disease may become concentrated in marginalized, harder-to-reach populations with low SES [8••]. This pattern echoes of the predictions of FCT: Improvements in living conditions, nutrition, access to care, and attention from public health authorities are likely to reach the well-off earlier than less-advantaged individuals. For TB, these effects may in fact be magnified. In the classic FCT model, care is received in response to the illness of each individual. In infectious disease field investigations, access to screening, treatment, and prevention occurs at the level of individual initiation as well as ascertainment by public health authorities via contact tracing. Consequently, if the efficacy and screening and treatment are greater among individuals who are both less likely to transmit, and to develop symptomatic disease upon infection, this may serve to increase SES disparities even while pushing community-level rates down.
Addressing the Fundamental Causes of TB
To move towards TB elimination, TB control research and policymaking must develop “fundamental interventions” [49] that target the upstream social determinants of health. However, because of their communicable nature, infectious diseases like TB present an additional complication to FCT: In addition to direct protection afforded to the person receiving treatment or screening, the advent of treatment and effective prevention for an infectious disease confers important indirect protective effects on untreated individuals. Those who are successfully treated or prevented from developing disease are subsequently unable to transmit to their contacts. Because these contacts are likely to be assortative, i.e., among individuals of the same social, economic, and demographic profile, the indirect benefits of treatment and prevention may then also be concentrated among individuals sharing characteristics of those who receive preferential access to treatment and prevention. This coupled diffusion of innovation and transmission is clearly a double-edge sword: In the most-advantaged populations, these dynamics may accelerate decline. But, in the highest-risk groups, the opposite is true: Where the accessibility of treatment, preventive therapy, and case-finding are lowest, transmission risks are likely to be the greatest.
Addressing the Role of SES Is Key to Successful Case-Finding and Intervention
As population prevalence decreases, the importance of each additional case for reaching the goal of TB elimination is increasing. Contact investigation has been consistently shown to be one of the most effective ways to find and treat cases in lower-incidence settings [50]. As the pool of cases becomes smaller, it may reflect increasingly poor and marginalized individuals, and their contacts, who are likely to represent individuals with a wide range of SES profiles. As a result, sustained attention to how SES impacts transmission, as well as the efficacy of case-finding, is critically necessary to provide an updated understanding of how to effectively target screening and intervention. Accomplishing this will require approaches that better account for individual- and population-level heterogeneity. Recent advances in spatial modeling and molecular genotyping techniques have been encouraging in this respect.
For example, a recent spatial analysis highlighted the need to incorporate information on the unstable housing situations of many individuals at highest risk of TB infection in the USA: In their analysis, Worrell et al. [51] created TB risk maps using the home locations of 198 TB cases in Fulton County, Georgia. Of these, 30% were among homeless individuals, and 50 cases reported 3 or more addresses at the time of diagnosis. When the multiple residential locations of these individuals were taken into account, the authors found that the area considered to be at highest risk of TB in the Atlanta area grew significantly in geographic size relative to a risk map including only a single address per individual. This highlights how the challenge of elimination is likely to scale inversely with prevalence, and why new theoretical perspectives and methodologies are critical to reach domestic and global public health goals.
A particular strength of molecular methods, such as MIRU-VNTR, spoligotyping, RFLP, and whole-genome sequencing (WGS), is that they may highlight transmission relationships between individuals without obvious epidemiological links, for example individuals who visited the same restaurant or bar but did not directly interact with each other (e.g., Klovdahl et al. [52]) or who had fleeting interactions in the community (e.g., Perri et al. [53]). In a recent review, Mathema et al. [54] highlighted the potential of whole-genome sequencing (WGS) to increase the efficacy of TB case-finding and intervention. Because of the relatively slow rate of TB mutation, chains of transmission may be difficult to detect using coarser genotyping techniques, such as MIRU-VNTR and spoligotyping. As a result, when a cluster of genomically similar TB cases share SES-related risk factors, it is difficult to determine whether they are related by direct transmission using such techniques. By allowing more rapid detection of recently infected individuals, these more finely pointed tools may allow recently infected individuals to be either detected at the earliest stage of infectiousness, or prevented from developing active disease via the administration of preventive therapy.
Move Towards a Systems-Based Approach
For much of its history, epidemiology has been a reductionist science, attempting to infer causality by isolating, via analysis or study design, the effect of one exposure on one health outcome. Many studies on the social determinants of TB reviewed here fit this description. However, it is becoming increasingly clear that effective interventions into the social determinants of health require research focused on the dynamic interplay between social institutions and systems of disease and health. And, studies of tuberculosis and other infectious diseases, where risk is dominated by the non-linear process of transmission, are particularly ill-suited to an entirely reductionist approach.
In this review, we have tried to highlight the ways in which the causal pathway between SES and TB is complicated by overlapping and mutually reinforcing risk factors. Specifically, the increased susceptibility to infection with MTB and the increased likelihood of progression to TB disease associated with low SES also increases the likelihood of exposure to MTB among the contacts of lower SES TB cases. These exposed individuals are then, as we have described above, more susceptible to infection and disease because of their socioeconomic status. Other feeedback loops between the social determinants and TB disease are well known in health disparities literature: Low levels of education and income, for example, lead to poorer health outcomes (i.e., TB disease) which in turn influence the ability to work or attend school and achieve higher levels of education and income [55]. In addition, there are feedback mechanisms linking TB and SES with other health outcomes, such as HIV [56, 57]. These complex dynamics suggest that research, and ultimately interventions, that lead to elimination of TB in the USA will need to focus less on the reductionist approach to epidemiology and instead adopt a holistic, systems-based view of the problem [8••].
We are far from the first to advocate for, or employ, a systems approach to TB control [48•, 58,59,60]. In fact, successful programs incorporating this view have been deployed in, for example, Peru [61], by addressing both the upstream (e.g., poverty, housing) causes and downstream (e.g., catastrophic costs due to lost wages) consequences of TB infection. Given that FCT suggests that SES disparities will only increase as we get nearer to the goal of elimination, it is imperative that TB control efforts in the US learn from their counterparts in other parts of the world who have successfully used a systems-based approach.
Conclusion
SES has long been understood to be a key factor underlying the distribution of TB, to the extent that it has become a truism of TB epidemiology. However, as US TB incidence rates have fallen, the center of gravity of the US TB epidemic has shifted as well, and a re-evaluation of how social determinants influence TB risk is necessary to move us towards elimination. This review has highlighted recent research documenting important shifts in the social patterning of TB in the USA. Yet, large gaps persist, particularly in explicating the precise pathways through which SES continues to operate to influence TB, and how these relationships will shift as prevalence continues to decline. At the same time, the proximal determinants of TB infection and disease have remained the same: These include neighborhood and housing environments, access to material resources, and exposure to psychosocial stress. Unpacking how the various dimensions of SES currently drive exposure to these more proximal risks in represents the most promising area of continued research. Indeed, the goal of US TB elimination is unlikely to be reached without targeted attention to socioeconomic drivers of the risk of infection and disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dubos RJ, Dubos J. The white plague: tuberculosis, man, and society. New Brunswick: Rutgers University Press; 1952.
McKeown T, Record RG. Reasons for the decline of mortality in England and wales during the nineteenth century. Popul Stud (NY). 1962;16:94–122. https://doi.org/10.1080/00324728.1962.10414870.
Zelner J, Muller C, Infection JF-E et al. Racial inequality in the annual risk of Tuberculosis infection in the United States. 1910–1933.
Roberts S. Infectious fear: politics, disease, and the health effects of segregation. Chapel Hill: Univ of North Carolina Press; 2009.
Stewart RJ, Tsang CA, Pratt RH, Price SF, Langer AJ. Tuberculosis—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317–23.
Link BG, Phelan JC. Understanding sociodemographic differences in health—the role of fundamental social causes. Am J Public Health. 1996 Apr;86(4):471–3.
Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:273–8. https://doi.org/10.15585/mmwr.mm6511a2.
•• Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45(4):928–52 This paper provides benchmarks for TB elimination in low-incidence countries, including actionable next steps for practitioners, researchers, and policy-makers. It highlights the multidisciplinary approach needed to move towards elimination.
Hill AN, Becerra JE, Castro KG. Modelling tuberculosis trends in the USA. Epidemiol Infect. 2012;140(10):1862–72.
Block JP, Subramanian SV. Moving beyond ‘food deserts’: reorienting United States policies to reduce disparities in diet quality. PLoS Med. 2015;12:e1001914. https://doi.org/10.1371/journal.pmed.1001914.
Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100:S186–96. https://doi.org/10.2105/AJPH.2009.166082.
Rubin M, Colen C, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100(6):1053–9.
Bennett D, Courval J. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care Med. 2008;177(3):348–55.
Noppert GA, Yang Z, Clarke P, Ye W, Davidson P, Wilson ML. Individual- and neighborhood-level contextual factors are associated with Mycobacterium tuberculosis transmission: genotypic clustering of cases in Michigan, 2004–2012. Ann Epidemiol. 2017;27:371–376.e5. https://doi.org/10.1016/j.annepidem.2017.05.009.
Holtgrave DR, Crosby RA. Social determinants of tuberculosis case rates in the United States. Am J Prev Med. 2004;26:159–62.
Di Gennaro F, Pizzol D, Cebola B, Stubbs B, Monno L, Saracino A, et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: a review. Tuberculosis. 2017;103:44–51.
• Bayer R, Castro KG. Tuberculosis elimination in the United States—the need for renewed action. N Engl J Med. 2017;377:1109–11. https://doi.org/10.1056/NEJMp1707387 This article highlights the complex social dynamics underpinning TB in the U.S. It brings to the forefront ethical concerns that must be considered when deciding on next steps for TB elimination in the U.S.
Siroka A, Ponce NA, Lönnroth K. Association between spending on social protection and tuberculosis burden: a global analysis. Lancet Infect Dis. 2016;16:473–9. https://doi.org/10.1016/S1473-3099(15)00401-6.
Singer PM, Noppert GA, Jenkins CH. Gaps in federal and state screening of tuberculosis in the United States. Am J Public Health. 2017;107:1750–2.
Davidow AL, Katz D, Ghosh S, Blumberg H, Tamhane A, Sevilla A, et al. Preventing infectious pulmonary tuberculosis among foreign-born residents of the United States. Am J Public Health. 2015;105:e81–8. https://doi.org/10.2105/AJPH.2015.302662.
Slopen M, Laraque F, Piatek AS, Ahuja SD. Missed opportunities for tuberculosis prevention in New York City, 2003. J Public Health Manag Pract. 2011;17(5):421–6.
Noppert GA, Wilson ML, Clarke P, Ye W, Davidson P, Yang Z. Race and nativity are major determinants of tuberculosis in the U.S.: evidence of health disparities in tuberculosis incidence in Michigan, 2004-2012. BMC Public Health. 2017;17(1):538. https://doi.org/10.1186/s12889-017-4461-y.
Grande K, Hunter P, Biedrzycki PA, Swain GR. Social determinants of health in public health practice: case study of rent stipends to augment tuberculosis cluster management. J Health Care Poor Underserved. 2014;25(4):1799–809.
Olson NA, Davidow AL, Winston CA, et al. A national study of socioeconomic status and tuberculosis rates by country of birth, United States, 1996–2005. BMC Public Health. 2012;12:365. https://doi.org/10.1186/1471-2458-12-365.
Zuñiga JA, Muñoz S, Johnson MZ, García AA. Mexican American men’s experience of living with tuberculosis on the U.S.–Mexico border. Am J Mens Health. 2016;10:32–8. https://doi.org/10.1177/1557988314555359.
Noppert GA, Clarke P. The modern profile of tuberculosis: developing the TB social survey to understand contemporary social patterns in tuberculosis. Public Health Nurs. 2018;35:48–55. https://doi.org/10.1111/phn.12372.
Craig G, Daftary A, Engel N, O'Driscoll S, Loannaki A. Tuberculosis stigma as a social determinant of health: a systematic mapping review of research in low incidence countries. Int J Infect Dis. 2017;56:90–100.
Stennis NL, Meissner JS, Bhavnani D, Kreiswirth B, Ahuja SD. Tuberculosis disease among Mexico-born individuals living in New York City, 2001–2014. Int J Tuberc Lung Dis. 2017;21(6):657–63.
Mindra G, Wortham JM, Haddad MB, Salinas JL, Powell KM, Armstrong LR. Tuberculosis among incarcerated Hispanic persons in the United States, 1993–2014. J Immigr Minor Health. 2017;19:982–6. https://doi.org/10.1007/s10903-016-0534-8.
Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Soc Sci Med. 2000;51:1143–61. https://doi.org/10.1016/S0277-9536(00)00016-2.
Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322(7):422–7.
Stead WW, Lofgren JP, Senner JW. Invited commentary: relative susceptibility of black Americans to tuberculosis. Am J Epidemiol. 1994;139:531–2.
Bor DH, Epstein PR. Pathogenesis of respiratory infection in the disadvantaged. Semin Respir Infect. 1991;6(4):194–203.
Cavalli-Sforza L, Menozzi P, Piazza A. The history and geography of human genes. Princeton: Princeton university press; 1994.
Stennis N, Trieu L, Ahuja SD, Harris TG. Estimated prevalence of tuberculosis infection among a New York City clinic population using interferon-gamma release assays. Open Forum Infect Dis. 2014;1(2).
O’Donnell MR, Chamblee S, von Reyn CF, Ellerbrock TV, Johnson J, March BJ, et al. Racial disparities in primary and reactivation tuberculosis in a rural community in the southeastern United States. Int J Tuberc Lung Dis. 2010;14(6):733–40.
Yuen C, Kammerer J, Marks K, Navin TR, France AM. Recent transmission of tuberculosis—United States, 2011–2014. PLoS One. 2016;11(4):e0153728.
Acevedo-Garcia D. Zip code-level risk factors for tuberculosis: neighborhood environment and residential segregation in New Jersey, 1985-1992. Am J Public Health. 2001;91:734–41.
Oren E, Narita M, Nolan C, Mayer J. Neighborhood socioeconomic position and tuberculosis transmission: a retrospective cohort study. BMC Infect Dis. 2014;14:227. https://doi.org/10.1186/1471-2334-14-227.
Prussing C, Castillo-Salgado C, Baruch N, Cronin WA. Geo-epidemiologic and molecular characterization to identify social, cultural, and economic factors where targeted tuberculosis control activities can reduce incidence in Maryland, 2004-2010. Public Health Rep. 2013;128(Suppl):104–14.
Bureau of Tuberculosis Control Annual Summary, 2016. Queens, NY; 2017.
Oren E, Koepsell T, Leroux BG, Mayer J. Area-based socio-economic disadvantage and tuberculosis incidence. Int J Tuberc Lung Dis. 2012;16(7):880–5.
Oren E, Narita M, Nolan C, Mayer J. Area-level socioeconomic disadvantage and severe pulmonary tuberculosis: U.S., 2000-2008. Public Health Rep. 2013;128:99–109.
Rodwell TC, Kapasi AJ, Barnes RFW, Moser KS. Factors associated with genotype clustering of Mycobacterium tuberculosis isolates in an ethnically diverse region of southern California, United States. Infect Genet Evol. 2012;12:1917–25. https://doi.org/10.1016/j.meegid.2012.08.022.
Myers WP, Westenhouse JL, Flood J, Riley LW. An ecological study of tuberculosis transmission in California. Am J Public Health. 2006;96:685–90. https://doi.org/10.2105/AJPH.2004.048132.
Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–16. https://doi.org/10.1093/phr/116.5.404.
The End TB Strategy. World Health Organization. 2015.
• Menzies NA, Cohen T, Hill AN, Yaesoubi R, Galer K, Wolf E, Marks SM, Salomon JA. Prospects for tuberculosis elimination in the United States: results of a transmission dynamic model. Am J Epidemiol. 2018. Published Online First: 14 May 2018. https://doi.org/10.1093/aje/kwy094. This study used a simulation model to examine the feasiblity of several different scenarios in the move toward TB elimination in the U.S.
Reich AD, Hansen HB, Link BG. Fundamental interventions: how clinicians can address the fundamental causes of disease. J Bioeth Inq. 2016;13:185–92. https://doi.org/10.1007/s11673-016-9715-3.
Anger HA, Proops D, Harris TG, Li J, Kreiswirth BN, Shashkina E, et al. Active case finding and prevention of tuberculosis among a cohort of contacts exposed to infectious tuberculosis cases in New York City. Clin Infect Dis. 2012;54(9):1287–95. https://doi.org/10.1093/cid/cis029.
Worrell MC, Kramer M, Yamin A, Ray SM, Goswami ND. Use of activity space in a tuberculosis outbreak: bringing homeless persons into spatial analyses. Open Forum Infect Dis. 2017;4(1). https://doi.org/10.1093/ofid/ofw280.
Klovdahl AS, Graviss EA, Yaganehdoost A, Ross MW, Wanger A, Adams GJ, et al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52:681–94.
Perri B, Proops D, Moonan PK, Munsiff SS, Kreiswirth BN, Kurepina N, et al. Mycobacterium tuberculosis cluster with developing drug resistance, New York, New York, USA, 2003–2009. Emerg Infect Dis. 2011;17(3):372–8.
Mathema B, Andrews JR, Cohen T, Borgdorff MW, Behr M, Glynn JR, et al. Drivers of tuberculosis transmission. J Infect Dis. 2017;216:S644–53. https://doi.org/10.1093/infdis/jix354.
Diez Roux AV. Complex systems thinking and current impasses in health disparities research. Am J Public Health. 2011;101:1627–34. https://doi.org/10.2105/AJPH.2011.300149.
Córdoba-Doña JA, Novalbos-Ruiz JP, Suárez-Farfante J, Andérica-Frías G, Escolar-Pujolar A. Social inequalities in HIV-TB and non-HIV-TB patients in two urban areas in southern Spain: multilevel analysis. Int J Tuberc Lung Dis. 2012;16(3):342–7. https://doi.org/10.5588/ijtld.11.0137.
Geng EH, Kreiswirth BN, Burzynski J, Schluger NW. Transmission trends for human immunodeficiency virus associated tuberculosis in New York City. Int J Tuberc Lung Dis. 2005;9:661–6.
Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–6. https://doi.org/10.1016/J.SOCSCIMED.2009.03.041.
Gardy JL, Johnston JC, Sui SJ, Cook VJ, Shah L, Brodkin E, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–9. https://doi.org/10.1056/NEJMoa1003176.
Wang CC, Zhu B, Fan X, Gicquel B, Zhang Y. Systems approach to tuberculosis vaccine development. Respirology. 2013;18:412–20. https://doi.org/10.1111/resp.12052.
Wingfield T, Boccia D, Tovar MA, Huff D, Montoya R, Lewis JJ, et al. Designing and implementing a socioeconomic intervention to enhance TB control: operational evidence from the CRESIPT project in Peru. BMC Public Health. 2015;15:810. https://doi.org/10.1186/s12889-015-2128-0.
Acknowledgements
The authors are grateful to Jeanne Sullivan Meissner at the New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control for her thoughtful feedback on the manuscript.
Funding
GN received salary support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number T32 HD091058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Grace A. Noppert reports grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development during the conduct of the study. Ryan E. Malosh reports grants from Multiparty Group for Advice on Science, outside the submitted work. Elizabeth B. Moran, Shama D. Ahuja, and Jon Zelner each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Social Epidemiology
Rights and permissions
About this article
Cite this article
Noppert, G.A., Malosh, R.E., Moran, E.B. et al. Contemporary Social Disparities in TB Infection and Disease in the USA: a Review. Curr Epidemiol Rep 5, 442–449 (2018). https://doi.org/10.1007/s40471-018-0171-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-018-0171-y