Abstract
During a survey of polypores in a Brazilian Atlantic rainforest fragment from Parque Estadual da Cantareira, São Paulo state, Brazil, 35 pileate species previously unregistered were found and identified, increasing to 52 the number of pileate species cited for this area. Abundisporus subflexibilis, Amauroderma praetervisum, Antrodia malicola, Antrodiella semisupina, Flabellophora parva, Skeletocutis roseola, Trametes membranacea, and Tyromyces atroalbus are new records from São Paulo state. Herbaria revision and taxonomic reevaluation of some records previously published for the area are discussed. Full descriptions, comments about morphology of the new records, and a complete identification key for the 44 confirmed species occurring in the locality are provided.
Similar content being viewed by others
Introduction
The polypores are fungi characterized by having a tubular hymenophore and being predominantly xylophilous, although they can also grow on soil or on living plants (Webster and Weber 2007). These fungi are important in nutrient and energy cycling processes due to their ability to degrade plant cell wall components like lignin (Kirk and Farrell 1987; Begon et al. 2006). Nevertheless, they are understudied in most tropical regions. It is estimated that there are approximately 20,000 species of polypores of which only 15 % is known (Hawksworth 2001).
Macroscopic characters of polypores are highly variable, the basidiomes can be resupinate or pileate, but there can also be transitions between both forms (Ryvarden 1991, 2004). The pileate sessile habit of the basidiomes is the most common in nature, apparently functioning as a compromise between the need for an efficient dispersal of the spores and the resistance to desiccation (Ryvarden 1991). Although these characters are greatly important in the morphological characterization of the taxa, the polypores do not form a monophyletic group, and several cases of morphological convergence exist (Hibbett and Binder 2002).
In the last years, several taxonomic works have been undertaken in São Paulo state improving the knowledge on this group (Bononi 1984a, b; Gugliotta et al. 2010; Abrahão et al. 2012; Gugliotta et al. 2013), but none has been focused on the diversity of Parque Estadual da Cantareira (PEC) which is considered the largest conservation unit in the world within an urban perimeter, with a significant area of the Brazilian Atlantic rainforest (~7.400 ha) (Secretaria de Estado do Meio Ambiente 2000).
Only two previous investigations reporting about polypores from the PEC were performed: (1) Hennings (1904, 1908) who described 21 species collected by Arsène Puttemans in 1898 and 1912, of which 18 were pileate; and (2) Fidalgo and Fidalgo (1957) who examined part of this material (14 specimens, all pileate). In this study, full descriptions, comments on morphology of the new records, and a complete identification key for the 44 confirmed species found in the region are presented.
Materials and methods
Specimens were collected from August 2010 to July 2012 in the Parque Estadual da Cantareira (PEC), (23°32′36′′S e 46°37′59′′W) located in the north end of the municipality of São Paulo, Brazil. The area is characterized by the presence of Dense Ombrophyllous Mountain Forests in different states of regeneration. The PEC is located at 750–1,215 m.a.s.l and has an area of 3.274 km2. The climate in the region is mesothermic tropical, with rainfall average of 1.570 mm year−1 (Secretaria de Estado do Meio Ambiente 2000).
The collection, herborization, and preservation of the material were carried out following techniques recommended by Fidalgo and Bononi (1984). The basidiomes were analyzed macro and micromorphologically according to the methods for the study of polypores (Núñez and Ryvarden 2001). For microscopic analysis, freehand sections of the basidiomes were mounted in microscope slides with a drop of 3 % KOH solution and 1 % phloxine solution; Melzer´s reagent was used to determine amyloid and dextrinoid (IKI+) reactions. Drawings of the microstructures were made with the assistance of a camera lucida. Colors are described according to Küppers (2002), the abbreviations and codes for the measurements are according Coelho (2005) with L m × W m = means of length and width, Q = range of length/width ratios, Q m = length/width mean, and n = x/y [x = number of measurements from a given number (y) of specimens]. Abbreviations of the authors of the species were made according to Kirk and Ansell (1992). All the specimens were deposited at SP herbarium (Instituto de Botânica, São Paulo, Brazil). Other materials of herbaria were also requested (E, ICN, K, and NY) for comparison, taxonomic reevaluation of some records previously published for the area, and confirmation of identifications [Thiers (Index Herbarium, continuously updated 2014)].
Results and discussion
Thirty-five additional species that have not been previously registered from the PEC were identified. Abundisporus subflexibilis (Berk. & M.A. Curtis) Parmasto, Amauroderma praetervisum (Pat.) Torrend, Antrodia malicola (Berk. & M.A. Curtis) Donk, Antrodiella semisupina (Berk. & M.A. Curtis) Ryvarden, Flabellophora parva Corner, Skeletocutis roseola (Rick ex Theiss.) Rajchenb., Trametes membranacea (Sw.) Kreisel, and Tyromyces atroalbus (Rick.) Rajchenb., were recorded for first time in the São Paulo state. It was only possible to confirm the presence of nine of the species recorded in previous studies [Datronia caperata (Berk.) Ryvarden, Fuscoporia gilva (Schwein.) T. Wagner & M. Fisch, Phellinus rimosus (Berk.) Pilát, Favolus brasiliensis (Fr.) Fr., Pycnoporus sanguineus (L.) Murrill, Rigidoporus microporus (Sw.) Overeem, R. ulmarius (Sowerby) Imazeki, T. elegans (Spreng.) Fr. and T. villosa (Sw.) Kreisel]; the other nine species were not found either in the field or in the herbaria collections to confirm their presence in this location [Coriolopsis polyzona (Pers.) Ryvarden, F. senex (Nees & Mont.) Ghob.-Nejh, Phaeolus subbulbipes (Henn.) O Fidalgo & M. Fidalgo, Phylloporia ribis (Schumach.) Ryvarden, P. varius (Pers.) Fr., T. versicolor (L.) Lloyd, T. pubescens (Schumach.) Pilát, Trichaptum biforme (Fr.) Ryvarden, and Tyromyces aquosus (Henn.) Ryvarden].
Identification key to polypores families
-
1.
Basidiospores double-walled, inner wall ornamented ……………………….. Ganodermataceae (KEY A)
-
1′.
Basidiospores with simple wall ………………... 2
-
2.
Basidiomes with xanthochroic reaction ……… …………………. Hymenochaetaceae (KEY B)
-
2′.
Basidiomes without xanthochroic reaction Other families (KEY C)
KEY A—Key to Ganodermataceae species
-
1.
Basidiomes sessile, basidiospores truncate ……… ………………………………...Ganoderma australe
-
1′.
Basidiomes stipitate, basidiospores ovoid, globose to subglobose, never truncated ……… ………...... 2
-
2.
Context heterogeneous with two bright parallel lines in the pileus and the stipe ……… Amauroderma praetervisum
-
2′.
Context homogeneous, without black lines in the pileus and the stipe ……… ……………………… 3
-
3.
Pilear surface of dermis-type, pores 3–4(−5) per mm ……… ……………………….. Amauroderma rude
-
3′.
Pilear surface of cortex-type, smaller pores [4–5(−6) per mm] ……… ……………………………….. 4
-
4.
Pilear surface dull, dark brown, basidiospores 7–8 × 5–7 µm ……… Amauroderma schomburgkii
-
4′.
Pilear surface shiny, dark reddish-brown, larger basidiospores ……… ………………………...... 5
-
5.
Basidiomes flexible, papery to leathery, basidiospores 8–8.5(−10) × 7–9(−9.5) µm ……… ……………………… Amauroderma exile
-
5′.
Basidiomes hard, subwoody to woody, basidiospores (13–)14–16 × (11)12.5–14(−15.5) µm ……… ……………………… Amauroderma pseudoboletus
Amauroderma exile (Berk.) Torrend, Brotéria, Ser. Bot. 18:142, 1920.
Polyporus exilis Berk., Hooker’s J. Bot. Kew Gard. Misc. 8:173, 1856.
Description in Furtado (1981).
The species is characterized by the reddish-brown basidiomes, the flexible pileus with shiny surface, not lacquered. It resembles A. schomburgkii (Mont. & Berk.) Torrend by the pileus surface of cortex-type. Amaurodema exile can be distinguished from A. schomburgkii by the presence of exudate on the pileus hyphae surface, the thinner hyphae in the context with thickened-walls and the slightly larger basidiospores [8–8.5(−10) × 7–9(−9.5) µm in the former and 7–8 × 5–7 µm in the latter] (Furtado 1981).
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 27-VI-2012, Motato-Vásquez V, Wetphalen M & Bolaños AC 235 (SP445607).
Amauroderma praetervisum (Pat.) Torrend, Brotéria, Ser. Bot. 18:131, 1920.
Ganoderma praetervisum Pat., Bull. Soc. Mycol. Fr. 5:78, 1889.
Description in Furtado (1981).
Amauroderma praetervisum is easily distinguished from other Amauroderma Murrill species by the presence of two very notable black lines in the context of stipe and pileus and also by the globose to subglobose basidiospores [(9–)10–12 × (8–)9–10 µm].
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 26-IV-2012, Motato-Vásquez V & Bolaños AC 177 (SP445608).
Amauroderma pseudoboletus (Speg.) J.S. Furtado, Revisão do gênero Amauroderma: 230, 1968.
Polyporus pseudoboletus Speg., Anales Soc. Ci. Argent. 16(6): 279, 1883.
Description in Furtado (1981).
Amauroderma pseudoboletus is characterized macroscopically by the reddish-brown pileus surface and the usually shiny and woody basidiomes. Microscopically, this species can be recognized by the pilear surface of cortex-type, the quite agglutinate hyphae, and by the globose to subglobose basidiospores [(13–)14–16 × (11–)12.5–14(−15.5) µm], yellowish, asperulate with distinct endosporic projections (Gugliotta et al. 2011). This species can be distinguished from the possibly related A. calcigenum (Berk.) Torrend by the larger pores [4–5(−6) per mm in the former and 1–2 per mm in the latter].
Examined specimens: BRAZIL. São Paulo: São Paulo, Serra da Cantareira, Horto Florestal, 28-III-1962, Furtado JS (SP61135, SP102715). PARAGUAY. 1879, (NY730911) (Holotype of P. pseudoboletus).
Amauroderma rude (Berk.) Torrend, Brotéria, Sér. Bot. 18: 127, 1920.
Polyporus rudis Berk., Ann. Mag. Nat. Hist. 3: 323, 1839.
Description in Furtado (1981).
Amauroderma rude has many macroscopic variations; the pileus surface varies in color and shape. Many pilei are described like infundibuliform from slightly convex or concentrically grooved to radially wrinkle. Microscopically, this species can be recognized by the subglobose, pale yellow, and finely ornamented basidiospores. Furtado (1981) recorded two varieties of this species, A. rude var. rude and A. rude var. intermedium Furtado. Ryvarden (2004) considered the species A. rude var. intermedium as an independent species [=A. intermedium (Bres. & Pat.) Torrend]. The macroscopic and microscopic characteristics of both species suggest that it is a single species, giving priority to A. rude.
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 23-X-2006, Karstedt F 802 (SP445605).
Amauroderma schomburgkii (Mont. & Berk.) Torrend, Brotéria, Sér. Bot. 18:140, 1920.
Polyporus schomburgkii Mont. & Berk., London J. Bot. 4:331, 1844.
Microporellus setigerus Corner, Beihefte zur Nova Hedwigia 86:119, 1987.
Description in Furtado (1981).
This species is characterized by the dark brown to black basidiomes with small, angular to circular pores [4–5(−6) per mm], the pileus surface of cortex-type, the globose to subglobose basidiospores (7–8 × 5–7 µm), hyaline, with thin endosporic projections, and the yellowish-brown skeletal hyphae (4–7 µm diam). Furtado (1981) recorded this species from Serra da Cantareira and suggested that this is the most common species of the genus in the Neotropics.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 16-II-2012, Capelari M & Oliveira JJS (SP445606), Serra da Cantareira, 1965, Singer R & Furtado JS (SP97660). GUYANA: (NY730960) (Holotype of P. schomburgkii).
Additional examined specimen: AMAZONAS: 14-VI-1947, Corner EJH (E151774) (Holotype of M. setigerus).
Ganoderma australe (Fr.) Pat., Bull. Soc. Mycol. France 5: 71, 1889.
Polyporus australis Fr., Elench. Fung. 1: 108, 1828.
Description in Ryvarden (2004).
Species with perennial basidiomes, black bands in the context, brown-colored pileus and whitish hymenial surface, reaching large dimensions (up to 50 cm diam). Torres-Torres et al. (2012) point out that the hard, dull, and thick crust (up to 0.1 cm) from the pilear surface may aid in the recognition of the species. Ganoderma australe is a taxon with problematic identity and belongs to the austral-applanatum species complex. Ganoderma applanatum (Pers.) Pat., is distinguished from G. australe by the smaller basidiospores, the context without black bands and by the distribution in temperate zones (Ryvarden 2004). So far, since the limits of austral-applanatum species complex are not well understood, the name G. australe will be applied to Neotropical species of the genus.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 21-X-2011, Motato-Vásquez V & Gugliotta AM 18 (SP445616), 29-XI-2011, Motato-Vásquez V 39 (SP445353), 31-XI-2011, Capelari M & Oliveira JJS 4608 (SP445604), 6-XII-2011, Motato-Vásquez V 69, 72 (SP445354, SP445403), 28-II-2012, Motato-Vásquez V 120, 123, 124 (SP445355, SP445356, SP445455), Serra da Cantareira, Horto Florestal, 15-XII-1954, sobre Acacia negra, Rodrigues A (SP108843).
KEY B—Key to Hymenochaetaceae species
-
1.
Basidiomes with lateral or central stipe ……… …………………… Phylloporia spathulata
-
1.
Basidiomes sessile ……… ……………… 2
-
2.
Monomitic hyphal system ……… Inonotus iodinus
-
2′.
Dimitic hyphal system ……… ………………….. 3
-
3.
Hymenial setae present ……… … Fuscoporia gilva
-
3′.
Hymenial setae absent ……… … Phellinus rimosus
Fuscoporia gilva (Schwein.) T. Wagner & M. Fisch., Mycologia 94(6):1013, 2002.
Boletus gilvus Schwein., Schriften Naturf. Ges. Leipzing 1:96, 1822.
Description in Ryvarden (2004).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 21-X-2011, Motato-Vásquez V & Gugliotta AM 22 (SP417971), 29-IX-2011, Motato-Vásquez V 40 (SP417972), 16-II-2012, Capelari M & Oliveira JJS 4683 (SP445350), 26-IV-2012, Motato-Vásquez V & Bolaños AC 169 (SP445348), 08-V-2012, Motato-Vásquez V 218 (SP445349), Serra da Cantareira, Horto Florestal, 27-I-1955, Pinheiro AN (109635), 13-I-1996, Marino MC (SP83915).
Inonotus iodinus (Mont.) G. Cunn., Bull. N. Z. Dep. scient. ind. Res. 78: 4, 1948.
Polyporus iodinus Mont., Annls Sci. nat., Bot. 16: 108, 1841.
Description in Ryvarden (2004).
This species is macroscopically characterized by basidiomes with flattened, dimidiate to flabelliform pileus; tomentose and concentrically sulcate and yellowish to dark brown pileus surface; black lines in the context and angular pores (3–6 per mm). Microscopically, it can be recognized by abundant straight hymenial setae and by cylindrical to ellipsoid basidiospores. Inonotus iodinus can be distinguished from the similar I. tabacinus (Mont.) G. Cunn., by the presence of leathery basidiomes which are brittle when dry, the smaller pores [3–6 per mm in the former and (7–)8–9 per mm in the latter], and the smaller basidiospores (4–4.5 × 2–3 µm in the former and 2.5–3.5 × 2.5–2 µm in the latter) (Núñez and Ryvarden 2000).
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 30-VII-2012, Motato-Vásquez V & Westphalen MC 267 (SP4117970).
Phellinus rimosus (Berk.) Pilát, Ann. Mycol. 38: 80, 1940.
Polyporus rimosus Berk., Lond. J. Bot., 4: 54, 1845.
Description in Ryvarden (2004).
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2012, Motato-Vásquez V 129 (SP445373).
Phylloporia spathulata (Hook.) Ryvarden, Synop. Fung. 5: 196, 1991.
Boletus spathulatus Hook., Syn. Pl. (Kunth) 1: 9, 1822.
Description in Ryvarden (2004).
This species is very variable in size and shape; however, it can be recognized by the stipitate basidiomes, with a thin black zone on the pileus, and the stipe that separates the tomentum of the context, and by the small-pigmented basidiospores. Phylloporia verae-crucis (Berk. Ex Sacc.) Ryvarden, another species that present stipitate basidiomes, differs from P. sphathulata by the slightly larger basidiospores [4–4.5 × 3–3.5 µm in the former and (2.5–)3–4 × 2–3 µm in the latter] (Wagner and Ryvarden 2002).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 26-IV-2012, Motato-Vásquez V & Bolaños AC 187, 188 (SP445374, SP445375).
KEY C—Key to species in other families
-
1.
Generative hyphae with simple septa ……… … 2
-
1′.
Generative hyphae with clamps ……… … 5
-
2.
Cistydia present, usually encrusted, embedded or slightly protruding, origin in the trama, 15–28.5 µm diam ……… ………………... Rigidoporus lineatus
-
2′.
Cystidia absent ……… ……………………….... 3
-
3.
Basidiospores allantoid (4–5 × 0.5–1 µm) ……… ………………………... Gloeoporus thelephoroides
-
3′.
Basidiospores subglobose to broadly ellipsoid ……… ………………………………………..... 4
-
4.
Basidiomes robust, hard, basidiospores subglobose to broadly ellipsoid (6–7 × 5–6 µm) ……… ............................ Rigidoporus ulmarius
-
4′.
Basidiomes thin, flexible when fresh, hard when dry, basidiospores subglobose (3–5 × (3–) 4–5 µm) ……… … Rigidoporus microporus
-
5.
Basidiomes distinctly stipitate ……… 6
-
5′.
Basidiomes effused-reflexed to sessile ……… 10
-
6.
Monomitic hyphal system ……… ...................... Flabellophora parva
-
6′.
Dimitic hyphal system ……… …………… 7
-
7.
Stipe dark brown to black ……… ……………… 8
-
7′.
Stipe cream to pale brown ……… ……………… 9
-
8.
Pores 0.5–1.5(−2) per mm, pileus surface cream to pale brown ……… ……….. Polyporus puttemansii
-
8′.
Pores 5–7(−10) per mm, pileus surface dark brown to black ……… ……………… Polyporus dictyopus
-
9.
Pores (4–)5–7 per mm, cilia present, hyphal pegs absent ……… ………………... Polyporus ciliatus
-
9′.
Pores 1–2 per mm, cilia absent, hyphal pegs present ……… ……………… ……… Favolus brasiliensis
-
10.
Monomitic hyphal system ……… ……………… 11
-
10′.
Di-trimitic hyphal system ……… ……………… 12
-
11.
Basidiomes soft and fragile, white to yellowish, becoming black when dry, pileus surface azonated, basidiospores ovoid to ellipsoid (3–3.5 × 2.5–3.5 µm) ……… ……………………….. Tyromyces atroalbus
-
11′.
Basidiomes resinous, cream, pink to brown, pileus surface with concentric zones, basidiospores cylindric to allantoid (3–5 × 1–1.5 µm) ……… ……………………….......... Gloeoporus dichrous
-
12.
Cystidia present ……… ……………………….. 13
-
12′.
Cystidia absent ……… ………………………... 16
-
13.
Skeletal hyphae dextrinoid, cystidia ventricose to clavate, embedded apically, 8–10 × 4–5 µm, basidiospores lacrimoid to ovoid-elongated (7.5–10 × 5–6 µm) ……….... Perenniporia martii
-
13′.
Skeletal hyphae not dextrinoid, basidiospores subglobose, ellipsoid to subcylindric ……… ……… 14
-
14.
Pileus surface grayish-brown to dark brown, pores 3–4 per mm, cystidia cylindrical, embedded apically, 12–14 × 3–5.5 µm, basidiospores ellipsoid to subcylindric (4.5–6 × 2–2.5 µm) ……… ………................... Trichaptum sector
-
14′.
Pileus surface white, cream to pale orange, basidiospores subglobose to broadly ellipsoid …..…… ......................... 15
-
15.
Pores 5–6 per mm, cystidia thick-walled, basidiospores 4.5–5 × 3.5–4.5 μm .... Junghuhnia undigera
-
15.
Pores 8–10 per mm, cystidia thin-walled, strongly dyed with phloxine and difficult to observe, basidiospores 2–3 × 2–2.5 µm ……… ..................... Flaviporus liebmannii
-
16.
Context white to cream or golden yellowish … 17
-
16′.
Context brown to reddish-orange ……… … 25
-
17.
Dimitic hyphal system ……… ……… … 18
-
17′.
Trimitic hyphal system ……… ………… 21
-
18.
Basidiospores dextrinoid with thickened walls ……… ............ Perenniporiella neofulva
-
18′.
Basidiospores not dextrinoid with thin walls ……… ………........................... 19
-
19.
Basidiospores allantoid, 4–6(−6.5) × 1.5–2 µm ……… ……….......... Antrodiella duracina
-
19′.
Basidiospores subglobose to broadly ellipsoid ……… ………............... 20
-
20.
Context heterogeneous, irregular pores 2–4 per mm, basidiospores broadly ellipsoid 3–4 × 2–3 µm ……… ………....... Antrodiella angulatopora
-
20′.
Context homogeneous, regular pores 8–10(−11) per mm, basidiospores subglobose to broadly ellipsoid, 2–3(−3.5) × 1.5–2.5 µm ……… ………............ Antrodiella semisupina
-
21.
Skeletal hyphae golden yellow ……… ………............. Coriolopsis rigida
-
21′.
Skeletal hyphae hyaline ……… ……….... 22
-
22.
Hymenophoral surface with irregular pores (2–3 per mm), angular to partially sinuous-dedaloid or lamellate ……… ………...... Trametes elegans
-
22′.
Hymenophoral surface with regular pores (3–8 per mm), angular to circular, more or less entire …… 23
-
23.
Pore surface vinaceous-brown ……… ...................... Trametes ochracea
-
23′.
Pore surface white, cream to beige ……… 24
-
24.
Pores angular 2–4 per mm, basidiospores cylindrical, 6.5–8 × 2–3(−3.5) µm ……… ..... Trametes villosa
-
24′.
Pores angular to circular 5–8 per mm, basidiospores ellipsoid to subcylindrical, (3–)3.5–4.5(5) × 2.5–3(−3.5) µm ……… ......... Trametes membranacea
-
25.
Dimitic hyphal system ……… …… 26
-
25′.
Trimitic hyphal system ……… …… 32
-
26.
Causing brown-rot ……… …… Antrodia malicola
-
26′.
Causing white-rot ……… ………....... 27
-
27.
Dextrinoid arboriform skeleto-binding hyphae, basidiospores yellowish-brown with slightly thickened walls ……… ....... Abundisporus subflexibilis
-
27′.
Not dextrinoid skeletal hyphae, basidiospores hyaline to faintly yellowish, thin-walled ……… 28
-
28.
Dendrohyphidia present, branched ……… 29
-
28′.
Dendrohyphidia absent ……… ………... 30
-
29.
Pore surface whitish to gray, irregular pores, sinuous to dedaloid, 1–2 per mm, basidiospores (5–)5.5–6 × (2–)2.5–3 µm ………... Fuscocerrena portoricensis
-
29′.
Pore surface vinaceous-brown, regular, hexagonal pores, (3–)3.5–5 per mm, basidiospores (8–)9–10.5(−11) × 3–4 µm ……… ……… Datronia stereoides
-
30.
Context heterogeneous, upper portion white and lower portion cartilaginous, basidiospores allantoid, 3–4.5 × (0.5–)1–2 µm ……… Skeletocutis roseola
-
30′.
Context homogeneous, basidiospores cylindrical to allantoid ……… ……………………………….. 31
-
31.
Skeletal hyphae dark fuliginous-brown, basidiospores cylindrical to allantoid, 3–4(−4.5) × 1–1.5 µm ……… ……………………….. Nigroporus vinosus
-
31′.
Skeletal hyphae yellowish-brown to dark golden brown, basidiospores cylindrical (6–)6.5–10 × 2.5–4 µm ……… ....... Datronia caperata
-
32.
Basidiomes orange to reddish-orange ……… ..................... Pycnoporus sanguineus
-
32.
Basidiomes of a different color, never orange to reddish ……… ………............. 33
-
33.
Crust lacquered dark-reddish to black on pileus surface, pores 7–8 per mm, basidiospores (5)5.5–7.5(−8) × 2–3 µm ………. Fomitella supina
-
33′.
Crust absent on the pileus surface, 1–2(−3) per mm, basidiospores (5–5–)6–7(−9) × 2.5–4 µm ……… ………………………...... Hexagonia papyracea
Abundisporus subflexibilis (Berk. & M.A. Curtis) Parmasto, Karstenia 40: 134, 2000.
Polyporus subflexibilis Berk. & M.A. Curtis, J. Linn. Soc., Bot. 10: 311, 1869.
Figures 1–4.
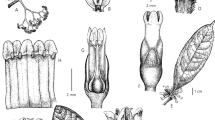
Basidiomes annual to perennial, pileate to effused-reflexed, sessile, woody, light after dry, solitary to imbricate. Pileus flattened, dimidiate, slightly convex to ungulate, widely adhered to the substrate, up to 4 × 2 × 0.5–1 cm. Pileus surface glabrous, smooth to concentrically zoned, pink (N20 A10 M50) to reddish-brown (N30 A99 M99) when fresh, and dark brown (N50 A99 M99) after dry. Margin acute, entire, rounded, concolorous with the pileus surface. Context homogeneous, dark brown, azonated, densely downy to woody, with a thin black cuticle on top, dark brown (N50 A99 M99), up to 0.3 cm thick. Pores surface vinaceous-brown (N60 A20 M70) to pale brown (N60 A99 M60). Regular pores, angular to circular, (5–)6–7 per mm. Tubes dark brown (N50 A99 M99), slightly paler that the context, up to 0.2 cm depth. Dissepiments thin and entire. Dimitic hyphal system. Generative hyphae with clamps, hyaline, with thin walls, 2–2.5 µm diam. Arboriform skeleto-binding hyphae, yellowish to yellowish-brown, with thickened walls, IKI+, 2.5–5 µm. Sterile elements absent. Basidia tetrasterigmate, clavate, 11–15.5 × 5–6 µm. Basidiospores ellipsoid, sometimes with one side flattened, yellowish-brown, with thick-walled and smooth, IKI−, 4–5(−5.5) × (2.5)3–3.5 µm (X m = 4.6 × 2.9 µm), n = 30/1, Q = 1.3–2 (Q m = 1.6).
Abundisporus subflexibilis has been synonymized with A. roseoalbus (Jungh.) Ryvarden (1998), a related species with smaller basidiospores [3.5–4 (−4.5) × 2.5–3 µm] and strongly swollen hyphae in KOH. Parmasto & Hallenberg (2000) conducted a statistical analysis of the basidiospores dimensions in A. roseoalbus and A. fuscopurpureus (Pers.) Ryvarden (species of paleotropical distribution), concluding that the dimensions of both species overlap, and should be treated as synonyms, leaving the possibility that the Neotropical representatives of A. roseoalbus could be considered a different species. In Brazil, many works have followed the criteria of Ryvarden (1998) and A. roseoalbus have been cited in Acre, Bahia, Pará, Paraná, and Rondônia states (Meijer 2008; Gugliotta et al. 2013). In this paper, after reviewing the type material of Poria roseoalba (Jungh.) Sacc. and P. subflexibilis and considering the results of Parmasto and Hallenberg (2000), A. subflexibilis and A. roseoalbus were considered as independent species; A. subflexibilis with neotropical distribution and A. roseoalbus with paleotropical distribution. Nevertheless, it is necessary to conduct a detailed study of the additional material collected in Brazil in order to access its identity with a combined morphological and molecular approach.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 24-VIII-2012, Motato-Vásquez V & Westphalen MC 277 (SP417944).
Additional examined specimens: BRAZIL. Rio grande do sul: Porto Alegre, Morro Santana, 14-IV-2005, Reck MA (ICN154154), 06-VII-2005, Reck MA (ICN154155). CUBA: Wright C 165 (K52122) (Holotype of P. subflexibilis). INDONÉSIA. Java Insulae: 1838, Junghuhn (NY742732) (Holotype of P. roseoalba).
Antrodia malicola (Berk. & M.A. Curtis) Donk, Persoonia 4(3): 339, 1966.
Trametes malicola Berk. & M.A. Curtis, J. Acad. Nat. Sci. Philad. 3: 209, 1856.
Description in Ryvarden and Gilbertson (1993).
This species is characterized by distinctly pale to dark brown basidiomes, effused-reflexed, sometimes with numerous imbricate pilei, dimidiate, flattened, with large pores. A. malicola could be confused with Trametopsis cervina (Schwein.) Tomsovsky, by its similar macroscopic morphology. However, the latter species has smaller basidiospores [7–9(−10) × 2.5–3 µm], larger pores [1–2(3) per mm], and cystidioles in the hymenium, and causes white-rot. A. malicola can also be distinguished from the similar A. albida (Fr.) Donk by the pore size and the different basidiomes color (brown basidiomes with 3–4 pores per mm in the former and white basidiomes with 1–3 pores per mm in the latter). In Brazil, A. malicola was previously recorded only from Paraná and Rio Grande do Sul states (Meijer 2008; Westphalen and Silveira 2013).
Examined specimen: BRAZIL. São Paulo: São Paulo, Serra da Cantareira, 1965, Singer R & Furtado JS (SP95448).
Additional examined specimens: BRAZIL. Rio grande do sul: São Francisco de Paula, CPCN Pró-Mata, 25-VI-2010, Westphalen MC 330/10, 331/10 (ICN154688, ICN154689).
Antrodiella angulatopora Ryvarden, Mycotaxon, 28(2): 525, 1987.
Description in Ryvarden (1987).
Antrodiella angulatopora is characterized by the effused-reflexed basidiomes, cream to beige, with larger and often irregular pores (2–4 per mm). It is distinguished from the very similar species Junghuhnia undigera (Berk. & M.A.Curtis) Ryvarden by the smaller, paler and flexible basidiomes, and by the presence of embedded cystidia. A. angulatopora was previously synonymized with Ceriporiopsis latemarginata (Rick) Rajchenb. (Rajchenberg 1987) since hyphal system can be interpreted as monomitic. Molecular evidence could contribute to clarify the taxonomic identity of A. angulatopora.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 6-XII-2011, Motato-Vásquez V 70 (SP417945), 30-I-2012, Motato-Vásquez V 112 (SP417946), 26-IV-2012, Motato-Vásquez V 175 (SP417947), 30-VII-2012, Motato-Vásquez V & Westphalen MC 261 (SP 417948).
Antrodiella duracina (Pat.) I. Lindblad & Ryvarden, Mycotaxon 71: 336, 1999.
Leptoporus duracinus Pat., Bull. Soc. Mycol. Fr. 18(2): 174, 1902.
Description in Lindblad and Ryvarden (1999).
This species has a wide variety of basidiomes shapes, but it can be recognized macroscopically by pileate basidiomes, sessile, dimidiate, concentrically zonated, cream to pale yellowish-brown pileus, with small pores [6–7(−8) per mm]. Microscopically, the allantoid basidiospores are characteristic. The nature of the hyphal system of this species needs to be clarified; some authors (Ryvarden and Johansen 1980; David and Rajchenberg 1985) have included this species in the genus Tyromyces P. Karst. due to their interpretation of the hyphal system as monomitic. In the examined material, the hyphal system was interpreted as dimitic, with monomitic context and skeletal hyphae restricted to the trama.
Examined specimen: BRAZIL. São Paulo: São Paulo, Serra da Cantareira, Vila Amália, Horto Florestal 11-I-1955, Rodrigues A (SP109546).
Antrodiella semisupina (Berk. & M.A. Curtis) Ryvarden, Prelim. Polyp. Fl. East Africa: 261, 1980.
Polyporus semisupinus Berk. & M.A. Curtis, Grevillea 1(4): 50, 1872.
Morphology of Antrodiella semisupina is highly variables, however, it can be recognized by the small basidiomes, dense, white to pale cream, the small pores, and the subglobose to ellipsoid basidiospores. A. duracina can be distinguished from A. semisupina by having a pale yellowish-brown to ochraceous basidiomes and allantoid basidiospores, [4–6(−6.5) × 1.5–2 µm].
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2012, Motato-Vásquez V 142 (SP445610), 28-II-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 225 (SP417949).
Coriolopsis rigida (Berk. & Mont.) Murrill, N. Amer. Fl. 9: 75, 1908.
Trametes rigida Berk. & Mont., Ann. Sci. Nat. Ser. 2, 11: 240, 1849.
Description in Ryvarden and Gilbertson (1993).
The yellowish effused-reflexed basidiomes, which can be easily separated from the substrata, is a diagnostic character of this species. It is distinguished from the Trametes Fr. species by the yellowish coloration of the context and by the golden context hyphae. Ryvarden and Johansen (1980) synonymized C. rigida with the pantropical species C. floccosa (Jungh.) Ryvarden. However, Gilbertson and Ryvarden (1986) maintained the separation of the two species, recognizing C. rigida as a species of Neotropical distribution.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vasquez V & Gugliotta AM 16 (SP417950), 29-XI-2011, Motato-Vasquez V 42 (SP417951), 30-I-2012, Motato-Vasquez V 114 (SP417952), 30-VII-2012 Motato-Vásquez V & Westphalen MC 270 (SP417953).
Datronia caperata (Berk.) Ryvarden, Mycotaxon 23: 172, 1985.
Polyporus caperatus Berk., Ann. Mag. Nat. Hist. 3: 391, 1839.
Description in Ryvarden and Johansen (1980).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 24-VIII-2011, Motato-Vásquez V 41 (SP417955), 28-II-2012, Motato-Vásquez V 117 (SP417956), 26-IV-2012, Motato-Vásquez V & Bolaños AC 170 (SP417957), 08-V-2012, Motato-Vásquez V 198 (SP417958), 28-II-2013, Motato-Vásquez V & Westphalen MC 269 (SP417959), 28-II-2013, Motato-Vásquez V & Westphalen MC 271 (SP417960), Serra da Cantareira, 5-V-1950, Teixeira AR (SP108809).
Datronia stereoides (Fr.) Ryvarden, Flora over kjuker: 42, 1968.
Polyporus stereoides Fr., Observationes mycologicae 2: 258, 1818.
Description in Ryvarden and Gilbertson (1993).
The granular nature of the dissepiments in this species is due to the abundant presence of the dendrohyphidia. This feature combined with the presence of a black line between the tomentum and the lower part of the context and the small regular pores allows the recognition of this species in the field.
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 26-IV-2012, Motato-Vásquez V & Bolaños AC 171 (SP417954).
Favolus brasiliensis (Fr.) Fr., Linnaea 5: 511, 1830.
Daedalea brasiliensis Fr. Syst. Mycol. (Lundae) 1: 332, 1821.
Description in Núñez and Ryvarden (1995).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 06-XII-2011, Motato-Vásquez V 66 (SP445623), 27-VI-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 234 (SP445624), 24-VIII-2012, Motato-Vásquez V & Westphalen MC 275 (SP445381), Serra da Cantareira, III.1902, Puttemans A 897 (SP141813, SP141810), 13-I-1966, Marino MC (SP83971).
Flabellophora parva Corner, Nova Hedwigia, Beih. 86:42, 1987.
Figures 5–8.
Basidiomata annual, stipitate to flabelliform, rarely solitary, imbricate pileus. Pileus circular to reniform. Pileus surface smooth to radially wrinkled, striated, white to cream (N00 A10 M00) when fresh to yellowish-brown (N20 A90 M50) when dry, elements type caulocystidia forming a pilear surface, clavate to mamillate, sparsely branched, with thin to slightly thickened wall, 13–15.5 × 3–5 µm. Stipe subpruinosus, thin, tubular, with fine hyphal fascicles on the surface, yellowish-brown (N20 A90 M50), up to 0.2–0.4 cm long. Margin thin, acute, entire when young to lightly lacerated in adult specimens, strongly involute when dry. Context thick and leathery in the lower portion and spongy in the upper portion, yellowish-brown, up to 0.1 cm thick. Pore surface white when fresh to pale yellow (N20 A60 M40) when dry. Pores regular, angular to circular, small, 10-14 per mm. Tubes easily separable from the context, yellowish-brown (N20 A90 M50), up to 0.1 cm depth. Dissepiments entire, thin, lacerates. Monomitic hyphal system in the context and dimitic hyphal system in the trama of the tubes. Generative hyphae with clamps, hyaline, thin to slightly thickened walls, 3–5 µm diam. Indistinct skeletal hyphae, with thickened to solid walls, straight, 5–8 µm diam. Basidia tetrasterigmate, clavate, 13–16 × 6–8 µm. Basidiospores subglobose to ellipsoid, hyaline, with a central drop of oily, thin and smooth wall, IKI−, 2.3–3 × 2–2.5 µm (X m = 2.7 × 2.3 µm), n = 60/2, Q = 1–1.3 (Q m = 1.2).
Flabellophora parva is characterized by basidiomes with pilei superimposed, although young specimens may present a single pileus. Microscopically, the species presents generative hyphae with clamps, different from the type species of the genus, F. superposita (Berk.) G. Cunn., that presents generative hyphae with simple septa (Cunningham 1965). Flabellophora obovata (Jungh.) Núñez & Ryvarden shares with F. parva the presence of a monomitic context, but differs by presenting wine to dark brown basidiomes, with pilear surface concentrically zonated and by wider basidiospores (3–4 µm diam) (Núñez & Ryvarden 2001).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 24-VIII-2012, Motato-Vásquez V & Bolaños AC 176 (SP417961), 24-VIII-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 249 (SP445671).
Additional examined specimens: BRAZIL. Rio Grande do Sul: São Leopoldo, 1905, Rick J 133 (SP124493, SP124493).
Flaviporus liebmannii (Fr.) Ginns, Canad. J. Bot. 58(14): 1584, 1980.
Polyporus liebmannii Fr., Nova Acta R. Soc. Scient. Upsal. 1: 59, 1851.
Description in Ginns (1980).
Macroscopically, the hard and resinous basidiomes, with zonated pilear surface, pale orange brown, slightly darkened after drying, and small pores are good features to recognize this species in the field. Microscopically, it can be recognized by the small basidiospores (2–3 × 2–2.5 µm), skeletal hyphae agglutinated and cystidia difficult to see, with thin-walled and oil content in dyed phloxine.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 26-IV-2012, Motato-Vásquez V & Bolaños AC 166, 178, 192 (SP417962, SP417963, SP417964), 27-VI-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 250 (SP417965), Serra da Cantareira, 29-V-1965, Teixeira AR & Furtado JS (SP95446).
Fomitella supina (Sw.) Murrill, Bull. Torrey Bot. Club 32(7): 365, 1905.
Boletus supinus Sw., Fl. Ind. Occ.: 1926, 1806.
Description in Gilbertson and Ryvarden (1986).
Fomitella supina is a common trametoid species characterized by the basidiomes flattened with a lacquered crust dark-reddish to black on the pileus surface and the brownish-gray pore surface with small circular pores. According to Gilbertson and Ryvarden (1986), Fomitella Murrill is differentiated from Fomitopsis P. Karst., by causing white-rot. Trametes cubensis (Mont.) Sacc. and Earliella scabrosa (Pers.) Gilb. & Ryvarden are neotropical species that also develop a reddish dull crust at the base of the pileus surface, but are differentiated from F. supina, by the whitish to cream yellow context and pore surface.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 08-V-2012, Motato-Vásquez V 205, 213 (SP417966, SP417967), Serra da Cantareira, 1901, Puttemans A 954 (SP141858), 1951, Zaidan-Nouer J (SP108813, SP109671).
Fuscocerrena portoricensis (Fr.) Ryvarden, Trans. Br. mycol. Soc. 79: 279, 1928.
Polyporus portoricensis Spreng. ex Fr., Elenchus Fungorum 1: 115, 1828.
Description in Gilbertson and Ryvarden (1986).
Fuscocerrena portoricensis is normally easy to recognize in the field due to the dark brown to vinaceous-brown pileus and the irregular hymenophore that becomes strongly split to dentate with age. Such characteristics are similar to those species of Hydnochaete Bres., but these species present a more rusty brown color, and microscopically present a generative hyphae with simple septa and straight dark brown setae.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vásquez V & Gugliotta AM 12 (SP417968), 26-IV-2012, Motato-Vásquez V & Bolaños AC 167 (SP417969), 24-VIII-2012, Motato-Vásquez V & Westphalen MC 273 (SP445364).
Gloeoporus dichrous (Fr.) Bres., Hedwigia 53: 506, 1912.
Polyporus dichrous Fr., Observa. Mycol. (Havniae) 1: 125, 1815.
Description in Ryvarden and Johansen (1980).
The basidiomes are variable from resupinate to pileate. This species is macroscopically characterized by the reddish-pink pore surface and microscopically by the monomitic hyphal system, generative hyphae with clamps, the cylindric to allantoid basidiospores (3–5 × 1–1,5 µm), and the continuous hymenium on dissepiments. Recent molecular analyzes suggest that G. dichrous groups with G. pannocincta (Romell) Niemelä. These species share the same type of septum and a particular gelatinous consistency when fresh (Núñez and Ryvarden 2001; Tomšovský and Ryvarden 2008). Further studies are necessary to clarify the taxonomic position of this species and the genus.
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 08-V-2012, Motato-Vásquez V 217 (SP445357).
Gloeoporus thelephoroides (Hook.) G. Cunn., Bull. N. Z. Dept. Sci. Industr. Res., Pl. Dis. Div. 164: 111, 1965.
Boletus thelephoroides Hook., Syn. Pl. 1: 10, 1822.
Description in Ryvarden and Johansen (1980).
The species is characterized by the gelatinized layer separating the context of the pore surface and by the monomitic hyphal system (Gilbertson and Ryvarden 1986). It is distinguished from G. dichrous by the presence of generative hyphae with clamps, and from G. taxicola (Pers.) Gilb. & Ryvarden by the ressupinate basidiomes, pore surface slightly reddish-purple, and larger pores (6–8 per mm in the former and 3–5 per mm in the latter) (Núñez and Ryvarden 2001).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 31-VIII-2006, Karstedt F & Capelari M 733 (SP445362), 04-XII-2008, Capelari M & Ramos LAS 4414 (SP445361), 20-IX-2011, Motato-Vasquez V & Gugliotta AM 01 (SP445358), 21-X-2011, Motato-Vasquez V 28 (SP445359), 29-XI-2011, Motato-Vásquez V (SP445360).
Hexagonia papyracea Berk., Ann. Mag. Nat. Hist. 10: 379, 1843.
Scenidium papyraceum (Berk.) Kuntz., Revisio generum plantarum 3: 516, 1898.
Description in Ryvarden and Johansen (1980).
This species is easily recognized by the flattened and flexible basidiomes, dimidiate, concentrically zoned in shades of reddish-brown, and by the hexagonal pores [1–2(−3) per mm].
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vasquez V & Bolaños AC 179 (SP445363), 09-II-2012, Capelari M & Oliveira JSS 4668 (SP445366).
Junghuhnia undigera (Berk. & M.A.Curtis) Ryvarden, Mycotaxon 20(2): 359, 1984.
Polyporus undiger Berk. & M.A. Curtis, J. linn. Soc., Bot. 10: 317, 1869.
Description in Ryvarden (1984).
Junghuhnia undigera can be recognized by the white, flexible, and effused-reflexed to dimidiate basidiomes, usually imbricate. Some larger specimens may be confused with species of the genus Trametes, however, this genus has coriaceous basidiomes, trimitic hyphal system, and lacks cystidia. Junghuhnia minuta I. Lindblad & Ryvarden also presents pileate and imbricate basidiomes, but differs from J. undigera by the very small pileate basidiomes that become brittle and resinous-hard after drying. In addition, J. minuta presents smaller basidiospores than J. undigera (2.5–3.5 × 2–2.5 μm in the former and 4.5–5 × 3.5–4.5 μm in the latter) and hyphal pegs in the hymenium (Westphalen et al. 2012).
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2013, Westphalen MC (SP).
Nigroporus vinosus (Berk.) Murrill, Bull. Torrey bot. Club 32(7): 361. 1905.
Polyporus vinosus Berk., Ann. Mog. Nat. Hist. 9: 195, 1852.
Description in Ryvarden and Johansen (1980).
This species is macroscopically characterized by the sessile and vinaceous-brown basidiomes. Nigrofomes melanoporus (Mont.) Murrill is similar in macromorphology, but differs from N. vinosus by having generative hyphae with simple septa (Núñez and Ryvarden 2000). Nigroporus vinosus could be confused with A. subflexibilis due to the similar coloration, however, it differs from the latter species by having hyaline, cylindrical to allantoid basidiospores [3–4(−4.5) × 1–1.5 µm in the former and 4–5(−5.5) × (2.5)3–3.5 µm in the latter] and unbranched skeletal hyphae.
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2012, Motato-Vásquez V 126 (SP445366).
Perenniporia martii (Berk.) Ryvarden, Norw. J. Bot. 19: 143, 1972.
Polyporus martius Berk., Hooker’s J. Bot. Kew Gard. Misc. 8: 198, 1856.
Description in Decock and Herrera-Figueroa (2000).
Perenniporia martii, originally described from Brazil, is characterized by the large perennial, woody, very hard basidiomes, dimitic hyphal system, with dextrinoid skeletal hyphae, cystidia apically encrusted, and lacrimoid basidiospores (7.5–10 × 5–6 µm). Perenniporia martii is very similar with P. latissima (Bres.) Ryvarden described originally from Java (Indonesia). Ryvarden and Johansen (1980) considered both synonymous, later Ryvarden (1988) recognized P. latissima as a distinct species with distribution limited to south Asia.
Examined specimens: BRASIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vasquez V 7 (SP445367), 21-X-2011, Motato-Vasquez V 20 (SP445368), 31-X-2011, Capelari M & Oliveira JJS 4609 (SP445369).
Perenniporiella neofulva (Lloyd) Decock & Ryvarden, Mycol. Res. 107(1): 94, 2003.
Polyporus neofulvus Lloyd, Mycol. Writ. 4 (Letter 60): 13, 1915.
Description in Decock and Ryvarden (2003).
Perenniporiella neofulva is macroscopically characterized by the pileate basidiomes, commonly white (when fresh) to pale brown, and microscopically by the dimitic hyphal system, branched arboriform skeletal hyphae, small and subglobose basidiospores, with thickened walls, slightly dextrinoid, and cyanophilous. Perenniporiella micropora (Ryvarden) Decock & Ryvarden can be distinguished from the similar P. neofulva by the thinner and more flexible basidiomes, the larger pores (5–7 per mm in the former and 9–11 in the latter), the narrow vegetative hyphae and the larger basidiospores (4.5–5 µm in diameter in the former and 3.5–4.5 µm in diameter in the latter). The morphologically similar species, P. contraria (Berk. & M.A. Curtis) Ryvarden can be distinguished from P. neofulva by the ellipsoid basidiospores and by the unbranched skeletal hyphae which are strongly dextrinoid in the context and the hymenial trama (Rajchenberg and Wright 1982; Decock et al. 2001).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2012, Motato-Vasquez V 140, 141 (SP445370, SP445371), 27-VI-2012, Motato-Vasquez V, Westphalen MC & Bolaños AC 237 (SP445372).
Polyporus ciliatus Fr., Observationes mycologicae 1: 123, 1815.
Polyporellus ciliatus (Fr.) P. Karst., Medden Soc. Fauna Fl. fenn. 5: 37, 1879.
Description in Núñez and Ryvarden (1995).
This species is characterized by the cream to pale brown pileus and the presence of cilia in the margin, predominantly in fresh specimens. The very similar P. tricholoma Mont. can be distinguished from P. ciliatus by the basidiomes with larger pores [(5–)6–7 per mm in the former and (4–)5–7 per mm in the latter] and the darker pileus surface. Krüger et al. (2003) mentioned the morphological, biological, and phylogenetic similarity between the species P. arcularius (Batsch) Fr., P. brumalis (Pers.) Fr., P. ciliatus, and P. tricholoma and suggested the possibility of being potentially hybrids. However, posterior studies detected intercompatibility between these species and obtained each species as closely related monophyletic clades with strong support (Krüger et al. 2004).
Examined specimens: BRASIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 21-X-2011, Motato-Vásquez V & Gugliotta AM 26 (SP445376), Capelari M & Oliveira JJS 4610 (SP445376).
Polyporus dictyopus Mont., Annls Sci. nat., Bot. 3: 349, 1835.
Melanopus dictyopus (Mont.) Pat., Essai taxonomique: 80, 1900.
Description in Núñez and Ryvarden (1995).
Polyporus dictyopus is characterized by the presence of a dark brown to black cuticle on the pileus surface and the stipe and by the small pores (Núñez and Ryvarden 1995). Polyporus dictyopus is macroscopically similar with P. melanopus (Pers.) Fr., however, it can be distinguished from the latter species by having smaller pores [5–7(−10) per mm in the former and 3–5 per mm in the latter] and smaller basidiospores [(5.5–)6–7 × 2.5–3.5 µm in the former and 8–11 × 3.5–4 µm in the latter]. In addition, P. melanopus is restricted to temperate zones (Silveira and Wright 2005).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 25-V-2006, Karstedt F 667 (SP445380), 08-V-2012, Motato-Vásquez V 212 (SP445378), Serra da Cantareira, 21-III-1903, Puttemans A 917 (SP141815), 4-XI-1952, Assunção A (SP108803).
Polyporus puttemansii Henn., Hedwigia 43: 200, 1904.
Polyporus guianensis var. puttemansii (Henn.) R.M. Silveira & J.E. Wright, Mycotaxon 93: 27, 2005.
Description in Silveira and Wright (2005) as P. guianensi var. puttemansii.
Silveira and Wright (2005) consider P. puttemansii as a variety of P. guianensis Mont. However, the material examined in this study differs enough morphologically from P. guianensis, and it is considered as a separate species. Specimens identified as P. puttemnasii are characterized by a cartilaginous and brittle basidiomes with large and angular pores [0.5–1.5(−2) per mm], a cream to pale brown pileus surface and a dark brown to black stipe. Basidiomes of P. guianensis are thicker, leathery, and hard; and have smaller pores (1–4 per mm). Polyporus leprieuri Mont. is also a species closer to P. puttemansii and P. guianensis, but it is distinguished from both species by the smaller pores (5–9 per mm) and by the production of rhizomorphs (Núñez and Ryvarden 1995).
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 28-II-2012, Motato-Vásquez V 119 (SP445380).
Pycnoporus sanguineus (L.) Murrill, Bull. Torrey Bot. Club 31(8): 421, 1904.
Boletus sanguineus L., Species Plantarum: 1646, 1763.
Description in Ryvarden and Johansen (1980).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 30-VII-2012, Motato-Vásquez V & Westphalen MC 260, 262 (SP441382, SP445383), Serra da Cantareira, 5-V-1950, Teixeira AR (SP108823).
Rigidoporus lineatus (Pers.) Ryvarden, Norw. J. Bot. 19: 236, 1972.
Polyporus lineatus Pers., Botanique (Nagpur) 5: 174, 1827.
Description in Ryvarden and Gilbertson (1994).
This species is characterized by the effused-reflexed basidiomes with orange pore surface. Microscopically, it is characterized by the presence of monomitic hyphal system, metuloid cystidia, often apically incrusted, and globose to subglobose basidiospores, usually with one large oil-drop. Rigidoporus lineatus is macroscopically very similar to R. microporus differing only by lacking cystidia (Ryvarden and Johansen 1980).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 29-XI-2011, Motato-Vásquez V 44, 47 (SP445384, SP445385), 30-I-2012, Motato-Vásquez V 108 (SP445386), 28-II-2012, Motato-Vásquez V 121, 127 (SP445387, SP445388), 26-IV-2012, Motato-Vásquez V & Bolaños AC 168 (SP445389), Serra da Cantareira, 13-I-1966, Marino MC (SP83964).
Rigidoporus microporus (Sw.) Overeem, Icon. Fung. malay. 1: 5, 1924.
Boletus microporus Sw., Prodr. Nov. Plant. Spec. India Occ.: 149, 1788.
Description in Ryvarden and Johansen (1980).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vásquez V & Gugliotta AM 3, 4 (SP445390, SP445391), 26-IV-2012, Motato-Vásquez V & Bolaños-Rojas AC 193 (SP445392).
Rigidoporus ulmarius (Sowerby) Imazeki, Bull. Govt Forest Exp. Stn Meguro 57: 97, 1952.
Boletus ulmarius Sowerby, Colored Figures of English Fungi 1: 39, 1797.
Description in Gilbertson and Ryvarden (1987).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 23-I-2009, Karstedt F 857 (SP445393), 25-VII-2006, Karstedt F 724 (SP445394), 13-I-1966, Marino MC (SP83970), VIII-1956, Teixeira AR, Fidalgo O & Fidalgo MEK (SP41404, SP41915).
Skeletocutis roseola (Rick ex Theiss.) Rajchenb., Nord. J. Bot. 7(5): 561, 1987.
Polystictus roseolus Rick ex Theiss., Denkschr. Akad. Wiss., math.-nat. Kl. 83: 239, 1911.
Figures 9–11.
Basidiomata annual, usually effused-reflexed to sessile, occasionally resupinate, solitary to imbricate or laterally connate, flexible consistency. Pileus flattened, dimidiate to flabelliform and thin, 5–8 × 2–5 × 0.1–0.4 cm. Pileus surface slightly zonated, azonated in some specimens, finely pubescent, beige (N20 A40 M20) to pale brown (N60 A99 M60). Margin sterile, thin, acute, entire, white to beige (N20 A40 M20) in contrast to the pore surface. Heterogeneous context, upper portion cottony, white, and lower portion more cartilaginous, dark beige (N20 A40 M20) to brown (N60 A90 M60), up to 0.2 cm thick. Pore surface grayish-brown (N60 A40 M30) to vinaceous-brown (N60 A20 M70). Pores regular, angular to circular, sometimes elongated, 6–7 per mm. Tubes shallow, up to 0.2 cm depth. Dissepiments thin, entire to slightly fimbriated. Dimitic hyphal system. Generative hyphae with clamps, hyaline, with thin to slightly thick-walled, some encrusted, 2–5 µm diam. Skeletal hyphae abundant in the upper portion of the context and the dissepiments, hyaline to slightly yellowish, straight, unbranched, thickened to solid wall, usually lumen absent, 2–5 µm diam. Cystidioles fusiform, smooth-walled to slightly encrusted, 7–9 × 3–4 µm. Basidia tetrasterigmate, slightly clavate, with basal clamps, 7–12 × 3–4 µm. Basidiospores allantoid, narrow, hyaline, thin-walled and smooth, IKI−, 3–4.5 × (0.5–)1–2 µm (X m = 3.8 × 1.6 µm), n = 90/5, Q = 2–3.2(−6) (Q m = 2.6).
Macroscopically, S. roseola can be recognized by the thin basidiomes with vinaceous hymenophore, pileus surface beige to pale brown, finely pubescent and duplex context, formed by an upper white cottony layer and a lower brown gelatinous layer. Microscopically, it is distinguished by the generative hyphae encrusted and very narrow allantoid basidiospores. The similar species S. amorpha (Fr.) Kotl. & Pouzar can be distinguished from S. roseola by the larger pores (3–4 per mm in the former and 6–7 in the latter) (Rajchenberg 1987).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 27-VI-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 240 (SP445395), 30-VII-2012, Motato-Vásquez V & Westphalen MC 268, 272 (SP445396, SP445397), Serra da Cantareira, Acampamento dos escoteiros, 08-XI-1951, Teixeira AR & Mendes MT (SP109629, SP109636).
Additional examined specimens: BRAZIL. Rio Grande do Sul: São Francisco de Paula, FLONA, 24-IV-2009, Westphalen MC 149-09 (ICN154327), 22-VI-2009, Westphalen MC 232-09 (ICN154328), 24-V-2010, Westphalen MC 328-10 (ICN154687).
Trametes elegans (Spreng.) Fr., Epicrisis Systematis Mycologici: 491, 1838.
Daedalea elegans Spreng., K. Svenska Vetensk Akad. Handl. 8: 51, 1820.
Description in Ryvarden and Johansen (1980).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 06-XII-2011, Motato-Vasquez V 67 (SP445398), 28-II-2012, Motato-Vasquez V 131 (SP445399), 27-VI-2012, Motato-Vasquez V, Westphalen MC & Bolaños AC 243 (SP445400), Serra da Cantareira, Horto Florestal, 20-XI-1953, de Morais N (SP108838).
Trametes membranacea (Sw.) Kreisel, Monogr., Cienc., Univ. Habana 16: 83, 1971.
Boletus membranaceus Sw., Prodr. Nov. Plant. Spec. India Occ.: 148, 1788.
Description in Gilbertson and Ryvarden (1987).
Trametes membranacea is characterized by the basidiomes with dimidiate to flabelliform pileus, finely velutinous surface, and small pores. The similar species T. versicolor can be distinguished from T. membranacea by the presence of tomentous pileus surface, with shades of brown and brilliant gray, and by the cylindrical, slightly larger, curved to allantoid, basidiospores (4.56.5 × 1.5–2.5 µm) in the former (Gilbertson and Ryvarden 1987) and the smaller, ellipsoid to subcylindric basidiospores [(3–)3.5–4.5(−5) × 2.5–3(−3.5) µm] in the latter.
Examined specimen: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 04-XII-2008, Capelari M & Ramos LAS 4419 (SP445401).
Trametes ochracea (Pers.) Gilb. & Ryvarden, North American Polypores 2: 752, 1987.
Boletus ochraceus Pers., Annl. Bot. (Usteri) 11: 29, 1794.
Description in Ryvarden and Gilbertson (1994).
Basidiomes of T. ochracea are usually much paler in color than those of T. versicolor less strongly zonate, and they lack layer seen in the upper context of the latter species (Ryvarden and Gilbertson 1994). The result of the molecular analyzes conducted by Justo and Hibbett (2011) based on a five-marker dataset, suggest a difficult separation of the species T. versicolor, T. ectypa (Berk. & M.A. Curtis) Gilb. & Ryvarden, T. ochracea and T. pubescens. Morphological separation of these taxa is also difficult due to the absence of diagnostic morphological characters for each species (Ryvarden and Gilbertson 1994). However, the intercompatibility test conducted by Tomšovský and Homolka (2004), suggests that they could be different biological species or at least being in an incipient part of the speciation process.
Examined specimen: BRAZIL. São Paulo: São Paulo, Serra da Cantareira, III-1903, Puttemans A (SP141856).
Trametes villosa (Sw.) Kreisel, Monogr., Cienc., Univ. Habana 16: 83, 1971.
Boletus villosus Sw., Fl. Ind. Occid. 3: 1923, 1806.
Description in Gilbertson and Ryvarden (1987).
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 20-IX-2011, Motato-Vasquez V, Gugliotta AM 8,9 (SP445469, SP445471), 21-X-2011, Motato-Vasquez V & Gugliotta AM 33 (SP445626), 29-XI-2011, Motato-Vasquez V 48 (SP445474), 30-I-2012, Motato-Vasquez V 106, 109 (SP445625, SP445627), 08-V-2012, Motato-Vasquez V 204 (SP445526), Serra da Cantareira, 14-VII-1905, Puttemans A 911 (SP141788), 05-XI-1952, Almeida F (SP109624), 11-I-1955, Rodrígues A (SP109500), 18-VII-1960, Eiten G & Eiten LT 2141 (SP61849), 13-I-1966, Marino MC (SP83935, SP83966).
Trichaptum sector (Ehrenb.) Kreisel, Monogr., Cienc., Univ. Habana 16: 84, 1971.
Boletus sector Ehrenb., Honrae Physicae Berolinenses 18: 6, 1820.
Description in Corner (1987).
This species is characterized by the small and flabelliform basidiomes, growing in numerous groups, with pale grayish-brown to vinaceous-brown pore surface and with generally lacerated dissepiments. Microscopically, it has encrusted cystidia and ellipsoid to cylindric basidiospores (4.5–6 × 2–2.5 µm).
Examined specimen: BRAZIL. São Paulo: São Paulo, Serra da Cantareira, próximo ao pico do Pavão, 29-V-1965, Teixeira AR & Furtado JS (SP95459).
Tyromyces atroalbus (Rick.) Rajchenb., Nord. J. Bot. 7(5): 558, 1988.
Polyporus atroalbus Rick, Botéria, ser. Ci. Nat. 4: 25, 1935.
Figures 12–14.
Basidiomata annual, pileate to effused-reflexed, sessile; margin, context and hymenophore gray to black with the contact. Pileus flattened, dimidiate, flexible when fresh to hard when dry, 8 × 6 × 3 cm. Pileus surface glabrous, azonate, white to yellowish-white (N20 A60 M40). Margin thin, acute, entire. Context homogeneous, white, fibrous when fresh, up to 1.5 cm thick. Pore surface white to yellowish-white (N20 A60 M40). Pores angular to circular, (2–)2.5–4(−5) per mm. Tubes concolorous with the pileus surface, up to 1.5 cm depth. Dissepiments entire. Monomitic hyphal system. Generative hyphae with clamps, hyaline, frequently branched, with thin to thickened-wall, 2–4 µm diam. Sterile elements absent. Basidia tetrasterigmate, clavate, 20–25 × 4–5 µm. Basidiospores ovoid to ellipsoid, hyaline, with thin and smooth-walled, IKI−, −3.5 × 2.5–3.5 µm (X m = 4.5 × 3 µm), n = 150/5, Q = 1.2–1.8(−2) (Q m = 1.5).
Tyromyces atroalbus is characterized by large basidiomes, white to yellowish when fresh and grayish to black to the contact, fibrous and flexible when fresh to hard when dry. Microscopically, the monomitic hyphal system, generative hyphae with conspicuous clamps and ovoid to ellipsoid basidiospores are diagnostic.
Examined specimens: BRAZIL. São Paulo: São Paulo, Parque Estadual da Cantareira, 7-XI-2011, Capelari M & Oliveira JJS 4626 (SP445630), 6-XII-2011, Motato-Vásquez V 59 (SP445628), 7-III-2012, Motato-Vásquez V 158 (SP445629), 27-VI-2012, Motato-Vásquez V, Westphalen MC & Bolaños AC 248 (SP445530), 30-VII-2012, Motato-Vásquez V & Westphalen MC 266 (SP445672).
References
Abrahão MC, Gugliotta AM, Bononi VLR (2012) Xylophilous Agaricomycetes (Basidiomycota) of the Brazilian Cerrado. Check List 8:1102–1116
Begon M, Towsend CR, Harper JL (2006) Ecology: from individuals to ecosystems. Blackwell Publishing, Oxford, pp 1–752
Bononi VLR (1984a) Basidiomicetos do cerrado da Reserva Biológica de Moji-Guaçu, SP. Rickia 11:1–25
Bononi VLR (1984b) Basidiomicetos do Parque Estadual da Ilha do Cardoso: IV. Adições às famílias Hymenochaetaceae. Stereaceae e Thelephoraceae. Rickia 11:43–52
Coelho G (2005) A Brazilian new species of Auriporia. Mycologia 97:263–267
Corner EJH (1987) Ad Polyporaceas IV. Nov Hedwig 86:1–265
Cunningham GH (1965) Polyporaceae of New Zealand. Bull N Z Dep Sci Ind Res 164:1–304
David A, Rajchenberg M (1985) Pore fungi from French Antilles and Guiana. Mycotaxon 22:285–325
Decock C, Herrera-Figueroa S (2000) Studies in Perenniporia. Navisporus ortizii, a synonym of Perenniporia martius, and a note on Navisporus and Perenniporia in Cuba. Cryptogam Mycol 21:153–162
Decock C, Ryvarden L (2003) Perenniporiella gen. nov., segregated from Perenniporia, including a key to neotropical Perenniporia species with pileate basidiomas. Mycol Res 107:93–103
Decock C, Herrera-Figueroa S, Ryvarden L (2001) Studies in Perenniporia. Perenniporia contraria and its presumed taxonomic synonym Fomes subannosus. Mycologia 93:195–203
Fidalgo O, Bononi VLR (1984) Técnicas de coleta, preservação e herborização de material botânico. Manual No.4, Instituto de Botânica, São Paulo
Fidalgo O, Fidalgo MEPK (1957) Revisão de Fungi São Paulensis. Arq Mus Nac 43:157–188
Furtado JS (1981) Taxonomy of Amauroderma (Basidiomycetes, Polyporaceae). Mem N Y Bot Gard 34:1–109
Gilbertson RL, Ryvarden L (1986) North American Polypores. Syn Fung 1:1–433
Gilbertson RL, Ryvarden L (1987) North American Polypores. Syn Fung 2:434–885
Ginns J (1980) The genus Flaviporus Murrill (Polyporaceae). Can J Bot 58:1578–1590
Gugliotta AM, Fonseca MP, Bononi VLR (2010) Additions to the knowledge of aphyllophoroid fungi (Basidiomycota) of Atlantic Rain Forest in São Paulo State, Brazil. Mycotaxon 112:335–338
Gugliotta AM, Poscolere GD, Campacci TVS (2011) Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP, Brasil. Fungos, 10: Ganodermataceae. Hoehnea 38:687–695
Gugliotta AM, Abrahão MC, Gibertoni TB (2013) Polyporales In: Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/floradobrasil/FB120646. Accesed 15 June 2013
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432
Hennings P (1904) Fungi S. Paulenses III a cl. Puttemans collecti. Hedwigia 43:197–209
Hennings P (1908) Fungi S. Paulenses IV a cl. Puttemans colecti. Hedwigia 48:1–20
Hibbett DS, Binder M (2002) Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proc R Soc Lond B 269:1963–1969
Justo A, Hibbett DS (2011) Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 60:1567–1583
Kirk PM, Ansell AE (1992) Authors of fungal names. A list of authors of scientific names of fungi with recommended standard forms of their names, including abbreviations. Index of Fungi Supplement. CAB International, Surrey
Kirk TK, Farrell RL (1987 ) Enzymatic “combustion”. The microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Krüger D, Hughes KW, Petersen RH (2003) Studies in Polyporus subg. Polyporellus: on congruence of three biological, morphological and phylogenetic species. In: Laflamme G, Bérubé JA, Bussières G (eds.) Root and Butt Rots of Forest Trees, pp. 14–23
Krüger D, Hughes KW, Petersen RH (2004) The tropical Polyporus tricholoma (Polyporaceae) taxonomy, phylogeny, and the development of methods to detect cryptic species. Mycol Prog 3:65–79
Küppers H (2002) Atlas de los colores. Editorial Blume, pp. 1–161
Lindblad I, Ryvarden L (1999) Studies in neotropical polypores 3. New and interesting Basidiomycetes (Poriales) from Costa Rica. Mycotaxon 71:335–359
Meijer AAR (2008) Macrofungos notáveis das florestas de Pinheiro-do-Paraná. Colombo, Embrapa Florestas
Núñez M, Ryvarden L (1995) Polyporus (Basidiomycota) and related genera. Syn Fung 10:1–85
Núñez M, Ryvarden L (2000) East Asian Polypores. Ganodermataceae and Hymenochaetaceae. Syn Fung 1:1–168
Núñez M, Ryvarden L (2001) East Asian Polypores Vol. II. Syn Fung 14:1–522
Parmasto E, Hallenberg N (2000) The genus Abundisporus (Hymenomycetes, Basidiomycotina). Karstenia 40:129–138
Rajchenberg M (1987) Type studies of Polyporaceae (Aphyllophorales) described by. J Rick Nord J Bot 7:553–568
Rajchenberg M, Wright JE (1982) Two new South American species of Perenniporia (Polyporaceae). Mycotaxon 15:306–310
Ryvarden L (1984) Type studies in the Polyporaceae. 16. Species described by J.M. Berkeley, either alone or with other mycologists from 1856 to 1886. Mycotaxon 20:329–363
Ryvarden L (1987) New and noteworthy polypores from tropical America. Mycotaxon 28:525–541
Ryvarden L (1988) Type studies in the Polyporaceae 20. Species described by G. Bresadola. Mycotaxon 33:303–327
Ryvarden L (1991) Genera of Polyporus. Nomenclature and taxonomy. Syn Fung 5:1–363
Ryvarden L (1998) African polypores—a review. Belg J Bot 131:150–155
Ryvarden L (2004) Neotropical Polypores. Part 1. Introduction Ganodermataceae and Hymenochaetaceae. Syn Fung 19:1–227
Ryvarden L, Gilbertson RL (1993) European Polypores. Vol I. Syn Fung 6:1–387
Ryvarden L, Gilbertson RL (1994) European Polypores. Vol. II. Syn Fung 7:1–743
Ryvarden L, Johansen I (1980) A preliminary flora of East Africa. Oslo, Fungiflora, pp 1–636
Secretaria de Estado de Meio Ambiente (2000) Atlas das Unidades de Conservação Ambiental do Estado de São Paulo. São Paulo, pp 1–64
Silveira RMB, Wright JE (2005) The taxonomy of Echinochaete and Polyporus s. str. in South America. Mycotaxon 93:1–59
Thiers B (2014) [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Disponível em: http://sweetgum.nybg.org/ih/. Accessed 15 May 2014
Tomšovský M, Homolka L (2004) Mating tests among geographically separated collections of the Trametes versicolor (Fr.) Pilát (Basidiomycetes, Polyporales) group. Nov Hedwigia 79:425–431
Tomšovský M, Ryvarden L (2008) Gloeoporus dichrous var. niger comb. nov. Mycotaxon 105:171–174
Torres-Torres MG, Guzmán-Dávalos L, Gugliotta AM (2012) Ganoderma in Brazil: know species and new records. Mycotaxon 121:93–132
Wagner T, Ryvarden L (2002) Phylogeny and taxonomy of the genus Phylloporia (Hymenochaetales). Mycol Prog 1:105–116
Webster J, Weber WS (2007) Introduction to fungi, 5th edn. Cambridge University Press, New York
Westphalen MC, Silveira RMB (2013) Pileate polypores from Araucaria Forests in Southern Brazil. Hoehnea 40:77–86
Westphalen MC, Reck MA, Silveira RMB (2012) The genus Junghunia in Brazil. Nov Hedwigia 94:209–220
Acknowledgments
The authors are grateful to the curators of the herbaria E, ICN, K, NY, and SP for the loan of types or original collections. The author Motato-Vásquez, V. also gratefully acknowledges the financial support received from the Programa Estudantes-Convênio de Pós-Graduação—PEC-PG, from CAPES/CNPq—Brazil. The author Pires, RM also gratefully acknowledges the financial support received from Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (12/25493-7). We also wish to thank Dr. Gerardo Robledo and MSc. Mauro Carpes Westphalen for the confirmation in the identification of some specimens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motato-Vásquez, V., Pires, R.M. & Gugliotta, A.M. Polypores from an Atlantic rainforest area in southeast Brazil: pileate species. Braz. J. Bot 38, 149–164 (2015). https://doi.org/10.1007/s40415-014-0109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-014-0109-7