Abstract
Background
Cost-of-illness data from empirical studies provide insights into the use of healthcare resources including both expenditures and the opportunity cost related to receiving treatment.
Objective
The objective of this systematic review was to gather cost data and relevant parameters for hepatitis B, pneumonia, meningitis, encephalitis caused by Japanese encephalitis, rubella, yellow fever, measles, influenza, and acute gastroenteritis in children in low- and middle-income countries.
Data Sources
Peer-reviewed studies published in public health, medical, and economic journals indexed in PubMed (MEDLINE), Embase, and EconLit.
Study Eligibility Criteria, Participants, and Interventions
Studies must (1) be peer reviewed, (2) be published in 2000–2016, (3) provide cost data for one of the nine diseases in children aged under 5 years in low- and middle-income countries, and (4) generated from primary data collection.
Limitations
We cannot exclude missing a few articles in our review. Measures were taken to reduce this risk. Several articles published since 2016 are omitted from the systematic review results, these articles are included in the discussion.
Conclusions and Implications of Key Findings
The review yielded 37 articles and 267 sets of cost estimates. We found no cost-of-illness studies with cost estimates for hepatitis B, measles, rubella, or yellow fever from primary data. Most estimates were from countries in Gavi preparatory (28%) and accelerated (28%) transition, followed by those who are initiating self-financing (22%) and those not eligible for Gavi support (19%). Thirteen articles compared household expenses to manage illnesses with income and two articles with other household expenses, such as food, clothing, and rent. An episode of illness represented 1–75% of the household’s monthly income or 10–83% of its monthly expenses. Articles that presented both household and government perspectives showed that most often governments incurred greater costs than households, including non-medical and indirect costs, across countries of all income statuses, with a few notable exceptions. Although limited for low- and middle-income country settings, cost estimates generated from primary data collection provided a ‘real-world’ estimate of the economic burden of vaccine-preventable diseases. Additional information on whether common situations preventing the application of official clinical guidelines (such as medication stock-outs) occurred would help reveal deficiencies in the health system. Improving the availability of cost-of-illness evidence can inform the public policy agenda about healthcare priorities and can help to operationalize the healthcare budget in local health systems to respond adequately to the burden of illness in the community.
Similar content being viewed by others
Few studies with primary data collection were conducted to assess the cost of vaccine-preventable diseases in low- and middle-income countries: there were none for measles, hepatitis B, rubella, or yellow fever. |
Cost estimates generated from primary data collection can provide a ‘real-world’ estimate of the economic burden of vaccine-preventable diseases. Additional information on whether common situations that may have influenced the application of official clinical guidelines (such as medication stock-outs) occurred, would help reveal deficiencies in the health system. |
Private healthcare is underrepresented. Estimating costs for private facility use offers a useful comparison with government-funded healthcare and provide insights for engaging private stakeholders in the universal health coverage strategy. |
1 Introduction
Vaccines are considered a highly cost-effective, public health intervention that can reduce the healthcare and household costs incurred by vaccine-preventable illnesses (VPD). To measure the scale of the economic burden from VPD, we rely on cost-of-illness (COI) studies to assess the costs associated with a specified illness and perspective [1, 2]. Cost-of-illness studies estimate the costs associated with treating and managing illnesses paid for by patients, governments, insurers, and charitable organizations. They also reveal costs borne by households to obtain healthcare, from travel and accommodations costs to the loss of income. As such, COI studies reflect a comprehensive view of the economic burden of disease and uncovers gaps in the health system that compromise equal access to healthcare.
In low- and middle-income countries (LMIC), defined by the World Bank lending groups [3], where governments rely on loans and external funding to finance public healthcare and immunization programs, COI studies are insightful to identify diseases that consume the most resources and aggravates inequalities in the population. Cost-of-illness studies relying on primary data collection, as opposed to modeling and secondary sources, would be best suited to capture all types of costs from different perspectives, using interviews or surveys of patients, caregivers, or healthcare professionals as well as medical records and administrative data.
Yet, prior reviews show that the number of studies focusing on diseases in LMIC with primary data collection paled in contrast to studies in high-income countries [4, 5]. For instance, of the 365 articles (1996–2006) reported in Akobundu et al. [5] only 20 focused on LMIC (and most using modeled data). We aim to bridge this apparent gap and review the costs associated with selected VPD in children in LMIC: hepatitis B, pneumonia, influenza, meningitis, encephalitis caused by the Japanese encephalitis virus (JE), rubella, yellow fever, measles, and acute gastroenteritis.
2 Methods
2.1 Literature Review
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines [6] and focused on studies that generated COI estimates from primary data collection, excluding those that generated COI estimates through modeling or secondary data sources. Studies that focused on infectious diseases that can potentially be prevented by vaccines including hepatitis B, pneumonia, meningitis, influenza, encephalitis caused by JE, rubella, yellow fever (YF), measles, and acute gastroenteritis (GE) in children aged under 5 years in LMICs were considered. For GE, we considered articles that presented data on GE either without specified etiology (e.g., diarrhea) or caused by rotavirus. We conducted an online literature search using three electronic databases: PubMed, Embase, and EconLit. We used a combination of controlled vocabulary and keyword terms that included the following concepts: (1) hepatitis B, pneumonia, influenza, meningitis, JE, rubella, YF, measles or GE, (2) cost data, (3) children, and (4) low- and middle-income countries (see Appendix 3 in electronic supplementary material (ESM)). We originally ran the search query for all infectious diseases reported on the World Health Organization’s (WHO’s) list before choosing to include only articles relevant to one of the nine diseases. We generated a list of keywords for LMIC based on the World Bank country classifications. We limited the search to peer-reviewed articles within the date range 2000–2016.
While we searched in English only, we included articles in French, Spanish, and Portuguese. All titles and abstracts relevant to our study were retrieved and searched for full text. Records from all databases were imported on 6 March, 2017. Potentially relevant articles published since then (as of 13 May 2020) are cited in the discussion. In addition to stand-alone COI studies, we included cost–benefit, cost-effectiveness, or cost-utility analyses if they produced COI estimates from primary data collection generated by the authors and not based on secondary sources.
2.2 Screening Process
Each article was examined by two reviewers over the four phases of the review process. Phase I examined the eligibility of each article by reviewing its title and abstract. For articles to be considered for inclusion, they had to: (1) be peer-reviewed articles published between 2000 and 2016; (2) provide cost data on VPDs in children aged under 5 years in LMICs; and (3) collect primary data. Phase II assessed eligibility based on the full text.
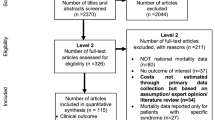
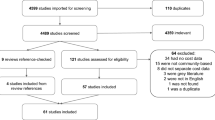
Instead of focusing on all childhood infectious illnesses, we narrowed our focus to nine VPDs: hepatitis B, all-cause pneumonia, all-cause influenza, all-cause meningitis, encephalitis caused by JE, rubella, YF, measles, and GE. This update to the screening process is reflected in the numbers of articles excluded in phase II (see Fig. 1).
Articles with conflicting reviewer decisions on eligibility were reviewed by the same two reviewers through a second round (labeled as “reconciliation”). The original response was removed so as not to make the reviewer aware of the decision made by the other reviewer.
2.3 Data Extraction
An article can produce more than one set of cost estimates, each set being associated with a specific perspective or scenario. For an article that reported cost estimates for the household and the healthcare system perspectives, comparing data from two different countries, and three VPDs (e.g., non-severe pneumonia, severe pneumonia, and meningitis), the authors may have presented 12 different sets of COI estimates: one per combination of parameters (two perspectives × two countries × three VPDs).
To highlight the multiple COI estimates reported per article, we disaggregated the articles into “sets”, effectively differentiating COI estimates as the authors report them. In this context, a “set” was defined as a scenario or a combination of parameters producing one distribution of costs, thus one cost estimate. Consequently, if there was more than one distribution of costs in an article, more than one set of costs was reported (Appendix 1 in ESM).
Data extraction was performed by two reviewers. Each set of costs extracted was checked by the second reviewer. Variances in data extraction between the two reviewers, such as the identification of sets of costs and the classification of the reported costs, were thoroughly discussed until agreement was reached. Variables and descriptions in the dataset were edited to reflect this.
2.4 Presentation of Cost Data
“Empirical COI evidence” was defined as the set of costs associated with an episode of the illness, estimated based on primary data collection [1, 2, 4, 5, 7]. To examine how cost estimates were structured and aggregated, we can refer to the Global Health Costing Consortium’s guidelines with the “intervention unit” cost associated with the cost per person diagnosed with the illness [8]. The selection of costs included when costing an illness is defined by an epidemiological approach (i.e., incidence or prevalence based) rather than as programmatic cost centered around the delivery of a specific intervention. It implies that a COI estimate can include costs outside the scope of an intervention (e.g., care provided at home, long-term productivity losses).
Costs are reported as service costs [8], except for the household perspective for which costs are classified as either direct or indirect costs [2]. Service costs include all costs associated with supplies such as medications for any services provided at the facility, and they integrate the healthcare facility operating costs and capital costs. For the household perspective, direct costs include the out-of-pocket payments incurred to access health services. Direct costs include medical (e.g., hospital charges, medications) and non-medical expenses (e.g., transportation to and from healthcare facilities, meals, and lodging for the caregivers). Indirect costs include the economic or opportunity costs incurred to receive or provide care including the costs of reduced productivity or lost time from paid employment resulting from illness or treatment [2]. A comprehensive list of costs is available in Appendix 4 in ESM.
The baseline cost (mean and/or median), sample size (n), and estimates of the error margin such as the confidence interval, interquartile values, and/or range are all presented [9]. The approach used to collect the cost data, either prospective or retrospective, is also presented. A summary of the cost estimates by perspective is presented in Tables 2, 3, and 4. All costs were converted in 2018 US$ using the average foreign exchange rates for the year [10] and the country’s consumer price index derived from the International Monetary Fund [11]. Furthermore, we discuss how the selected articles compare in light of the recommendations of prior reviews of COI studies, more particularly Clabaugh and Ward [4].
3 Results
The search yielded a total of 12,792 unique articles after duplicates were removed (Fig. 1). After reconciliation, 405 articles moved on from the first phase of screening. Moving on to the full-text review (phase II), we narrowed the diseases of interest to nine diseases: articles focusing on other diseases were still included in the review. Reviewers examined the full text of 396 articles (nine articles did not have the full text available, they were likely poster abstracts) and found that 114 articles did not present costs generated empirically and 245 articles did not present data on any of the nine diseases of interest. We made a final selection of 37 articles. The level of inter-reviewer agreement (Cohen’s kappa) was 68.1% (substantial agreement) by the end of the screening process [12].
3.1 Countries, Illness, and Populations
From the 37 articles, we drew 267 different sets of costs. Five of the nine VPDs were identified from the 267 sets of costs: GE (116 sets, 43%), pneumonia (121, 45%), meningitis (22 sets, 8%), JE (three sets, 1%), influenza (two sets, 1%), and other illnesses (three sets, 1%). In the latter, two sets only specified “other” and “very severe disease” related to pneumococcal infection and one specified acute otitis media. We found no estimate for hepatitis B, measles, rubella, and YF in children. Children between the age of 1 and 59 months were the main age group under study (218 sets, 81%), followed by children aged 0–3 years (35 sets, 13%). Children aged older than 5 years were also included in six sets of costs, 2% (Table 1).
The largest portion of these costs came from countries in Sub-Saharan Africa (97 sets, 36%), followed by South Asia (80 sets, 30%), East Asia and Pacific (67, 25%), Latin America and the Caribbean (16 sets, 6%), Europe and Central Asia (four sets, 2%), and the Middle East and North Africa (three sets, 1%). Based on Gavi’s 2018 Annual Progress Report [13], most sets came from countries in preparatory (75 sets, 28%) and accelerated transitions (also 75 sets, 28%), followed by countries initiating self-financing (59 sets, 22%) and non-Gavi-eligible countries (51 sets, 19%). Indonesia was the only fully self-financing country with a COI study (seven sets, 3%).
Most COI evidence was generated in countries where publicly funded healthcare was available. The most commonly reported perspectives were the government perspective where we found 96 sets of costs (36%) and the household perspective (79 sets, 30%) for which most of the data collection was performed in public healthcare facilities. Studies that adopted the healthcare and the household perspectives also adopted a societal perspective in 47 sets of costs (18%). Thirty sets of costs (11%) were reported from the perspective of private healthcare providers without further details on whether the costs were transferred to users or other sources of revenue. Eleven sets of costs (4%) took a healthcare provider perspective, not differentiating what was paid by the government and by the private sector. One article reported costs borne by health insurance, hence taking a third-party payer perspective in four sets of the costs (2%).
Most sets of costs were associated with COI in urban settings (127 sets, 48%). Eighty-three sets (31%) were associated with rural settings and 57 sets with mixed urban and rural settings (21%). In our selected articles, each set of costs could combine costs for more than one facility level: 164 sets of costs (61%) included costs from tertiary healthcare facilities, 81 (30%) from secondary healthcare facilities, and 93 (35%) from primary healthcare facilities.
3.2 Scope and Methods
Of the 37 articles, 11 (30%) integrated a COI component with primary data collection within a larger study. Five of 37 papers were cost-effectiveness analyses, four were burden of disease studies, and two were randomized controlled trials. The remaining 26 articles were stand-alone COI studies. These COI studies took an incidence-based cross-sectional approach, defining the COI around the healthcare facility visit for an acute episode of the illness. While they focused on the acute episode of the illness, most sets of costs adopting the household perspective included a follow-up period, 7–14 days after the initial episode.
To identify VPD cases, 23 articles reported costs with laboratory-confirmed cases (GE, meningitis, JE) and nine with radiology-confirmed cases (pneumonia). Among these, six studies used both laboratory testing and radiology. Eleven articles relied on clinical assessment alone to identify cases.
Twenty-one articles (57%) took a prospective approach to data collection, seven (19%) took a retrospective approach, and nine (24%) combined prospective and retrospective approaches. Costs from the household perspective were always estimated prospectively as these relied on caregiver responses. Ten articles (27%) included caregiver interviews. Most of them (eight articles, 22%) performed a follow-up interview 7–14 days after the initial interview. One article performed interviews 6 weeks later [14], another 5–12 months afterward [15].
Among the articles assessing the cost of GE (19 articles), half of them used an established costing method. Eight used the WHO guidelines to estimate the economic burden of diarrhea published in 2005 [14, 16,17,18,19,20,21,22], one used the WHO guideline for cost analysis in primary healthcare published in 1994 [23], and one used unpublished WHO guidelines cited as “WHO (U Griffiths, R Rheingans, D Walker, unpublished data)” [24]. One article focusing on pneumonia and meningitis used the national guideline from the Ministry of Finance (Vietnam) to estimate capital costs [25]. None reported a qualitative component (expert consultations) to identify potential costs for households and the healthcare system.
3.3 Types of Costs
All 37 articles presented direct medical costs. Twenty-three (62%) also presented direct non-medical costs and 24 (65%) presented indirect costs. Twenty-two articles presented all three types of costs. Direct medical costs included medications and medical procedures, and most articles presented such medical costs aggregated.
Five articles (14%) included overhead costs [26,27,28,29] and two (5%) added discounted capital costs [25, 29]. Four articles [25,26,27, 29] counted them as part of the medical costs, while one [28] included them as part of an “indirect cost” from the provider perspective.
Direct non-medical costs included the cost of transportation (21 of 37 articles), meals during the facility visit (9 of 37), caregiver accommodations (3 of 37), and other costs related to childcare (8 of 37) such as diapers, visitors’ gifts, and transportation for non-caregivers. Four articles presented aggregated direct non-medical costs only and one article aggregated all direct medical and non-medical costs as a “total cost of admission”.
Indirect costs were based on income loss for the caregiver(s) (18 of 37 articles) and time loss for the caregiver because of disability (1 of 37 articles). The other four articles presented only an aggregated indirect cost value and did not describe cost composition. All indirect costs were estimated through a human capital approach, considering the productivity loss of caregivers. Among the articles reporting income loss, six articles estimated income loss for the surveyed caregiver only [15, 17, 24, 26, 30, 31], eight estimated it for both the father and the mother of the sick child [18, 19, 22, 32,33,34,35,36], and three assessed income loss for all reported caregivers [21, 27, 37]. One article did not disclose whose income was lost [38].
Most articles reported the costs during the year of data collection or the year following data collection, without any correction for inflation. One article [26] used unpublished costs originally collected in 1998–2000 and adjusted them to the 2010 national currency value. Several articles [18, 19, 24, 38,39,40] collected costs over several years and did not specify the year of the currency value; we assumed the year of the currency was the starting year for data collection to correct for the missing base year.
3.4 Cost Estimates
Direct medical, non-medical, and indirect costs per episode for inpatient care were greater than for outpatient care for the household perspective (Table 2). There was no apparent trend between these costs and country income status. For pneumonia and GE episodes, household direct medical costs had a range of $3.52–$125.39 per hospitalized case and $0–$53.87 per ambulatory case. For JE, the medical costs ranged between $577.65 and $1268.84. Non-medical costs were similar across country income statuses and diseases, $1.21–$28.29 (inpatient), and $0–$8.94 (outpatient), with an exception for meningitis where they were much higher: $28.78–179.46 (mixed inpatient/outpatient). Ranges of reported indirect costs were similar between diseases and overlapped across country income statuses with larger variations with higher income statuses: $11.21–$41.31 (low income), $2.25–$90.78 (lower-middle income), and $0.55–$214.55 (upper-middle income) (Tables 2, 3, and 4).
In addition to differences by the type of care provided, government costs per hospitalized episode increased with higher country income status (Table 3). Governments spent $46.76–$84.95, $130.86–$442.54, and $205.07–$6623.99 per hospitalized episode across all diseases in low-, lower-middle-, and upper-middle-income countries, respectively. In most articles including both the household and the government perspectives, governments faced greater costs per episode of illness than households. In Le et al., the government spent more than households on medical care for an episode of pneumonia and meningitis in Vietnam; however, when including non-medical costs, the household direct costs exceeded those of the government [37]. There were strong differences in societal costs across diseases and types of care (Table 4).
We examined the share of the costs from the household perspective and focused on 27 sets of costs (from nine articles) that looked at households using public healthcare facilities, who reported direct medical, direct non-medical, and indirect costs (Appendix 2 in ESM). Across diseases and settings, the proportion of non-medical and indirect costs outweighs the medical costs. Non-medical costs dominate (54%) other costs over outpatient care, while indirect costs take the highest share (43%) of the total cost for inpatient care. Medical costs made 21% of the cost of hospitalized pneumonia and between 1% and 14% for outpatient pneumonia, while non-medical and indirect costs estimated at 62–86% and 10–15%, respectively. For GE, medical costs made 12–44% of the cost of a hospitalized case with non-medical (14–16%) and indirect costs (41–72%). One set reported that households had no out-of-pocket expenses for outpatient GE, facing only indirect costs.
Three sets of costs included all three types of costs for households using private healthcare facilities, representing inpatient care only. The average proportion of direct medical costs (69%) is higher than that of direct non-medical (16%) and indirect costs (15%) (Appendix 2 in ESM).
3.5 Economic Burden on Households
In addition to the breakdown of the burden of costs borne by the households, we examined whether authors took the additional step to interpret COI estimates in relation to income or total expenditure of the household or government, which we defined as economic burden measures.
In this review, 18 articles (60%) described the economic burden of illness with 45 economic burden measures. These composite measures contain a variety of numerators and denominators as demonstrated in Table 5.
The result shows that the percentage of COI as a percentage of household expenditure ranges from 43% to 83%. Cost of illness as a percentage of household income ranges from 0.39% to 1000%, depending on the illness and the choice of numerator and denominator. Cost of illness as a percentage of government per capita expenditure falls between 3.5 and 82%.
3.6 Funding
The main source of funding for the studies identified in this review came from multi-lateral agencies (WHO, UNICEF), non-government organizations, and philanthropies (22 articles, 59%), followed by governments and public organizations (16 articles, 43%) and the private sector (eight articles, 22%). Four articles did not disclose any source of funding [27, 28, 34, 35].
4 Discussion
From 2000 to 2016, only 37 articles were found to produce COI estimates based on primary data collection for potentially pediatric VPD. Clabaugh and Ward [4] found 52 articles focusing on COI in the USA published between 2000 and 2004 collecting data from insurance claims and facility administration databases, including charges applied to patients and caregivers. Akobundu et al. [5] found 365 articles presenting COI estimates generated from primary data collection or by modeling existing cost estimates from the literature between 1996 and 2005. Their review included 20 articles focusing on LMIC, albeit none of which fulfilled the selection criteria for our review: different diseases of interest (epilepsy, malaria, human immunodeficiency virus [HIV]) and/or using modeled data. The scarcity of electronic and standardized patients’ records in LMIC implies a heavy reliance on a prospective approach to costing, implying that the researchers must conduct their own data collection—understandingly, an expensive endeavor.
The cost estimates for different illnesses were challenging to compare owing to the wide range of healthcare costs included, diverse disease definitions, and unclear perspectives. Building upon the work of Rice [41] and Hodgson and Meiners [7], Clabaugh and Ward made several recommendations to improve the reliability of COI studies [4]. In our selection of COI studies, most of these recommendations were met and we review here what is still missing.
Study perspective in their review, Clabaugh and Ward [4] first recommended to disclose the economic perspective considered and ensure its coherence with the costs included in the COI estimate. There was one article where the costs reported do not correspond with the study perspective [42]. The societal perspective should combine both the provider and the caregiver’s perspectives, and include indirect costs related to productivity loss.
Definition and comprehensiveness of cost components in parallel, researchers must identify and include all the components of care relevant to the treatment of the illness [4, 41]. All studies integrated the most tangible costs related to care such as medications, diagnostic tests, and non-medical costs. Two studies distinguished themselves by including capital and overhead costs [18, 29]. In Burke et al. [18], the researchers suggested that the real COI falls between the costs in the public sector and the costs from the private clinics, as the cost estimates for the former did not include administration costs, operation costs, and the depreciation of the infrastructure. While capital and overhead costs are only a small share of the COI estimate, they are not negligible.
Additionally, national guidelines provide strict rules for the care of these diseases, but local practices may diverge because of a lack of resources or oversight [43]. In most of the selected articles, item costs and utilization were drawn from medical health records and interviews, providing an accurate perspective on the ‘real-world’ COI. However, none of the articles explicitly discussed whether the care provided corresponded to the guidelines. Mathew et al. [44] mentioned that an increase in COI is linked to increased quality of care, yet no article explored whether lower COI is related to worse health outcomes. To understand how healthcare is provisioned and organized, researchers should conduct a brief qualitative study or consultations that complement quantitative data. While most recognized missing or unforeseen costs as a limitation, none of the selected articles reported conducting such a study proactively.
Disease definition Clabaugh and Ward [4] expressed the need to standardize the use of the “second” diagnosis received by a patient to confirm the case and its inclusion in the COI study. As opposed to the “first” diagnosis performed through the initial clinical assessment, the second diagnosis usually follows additional laboratory investigations and is, therefore, more reliable. Laboratory testing allows for more stringent eligibility criteria to be applied, where only cases with a specific disease etiology are included. Such a narrow disease definition may allow researchers to associate the economic data with specific interventions like vaccines. Over half of the articles used laboratory tests and radiology to ascertain the etiology of the disease.
The presence of comorbidities, particularly HIV/AIDS or malaria, is scarcely discussed in the selected articles. The articles were not clear on whether researchers included cases of children who had comorbidities or were immunocompromised. Only two articles [26, 45] focused on HIV and examined the differences in costs between immunocompromised and immunocompetent patients for acute lower respiratory tract infections. Note, however, that our review excluded articles that focused on HIV solely or on HIV and other infectious diseases than the nine diseases of interest.
Time horizon once the disease case is identified, the scope of the costs and the reliability of their reporting depends on the chosen time horizon [8]. All the selected articles opted for an incidence-based approach, associating the COI estimate to an episode of the illness rather than to a yearly average. The lack of studies taking a prevalence-based approach is not surprising as we focused on infectious illnesses characterized by the presence of an acute phase requiring treatment. It is possible to capture entire episodes of the illness even with such short periods for data collection. Furthermore, eight articles followed the patient beyond the acute phase of the illness. Doing so allowed the researchers to review the COI estimates reported during the initial interview and consolidate them with costs that occurred thereafter. Follow-ups can reduce recall bias and help obtain a more comprehensive set of the costs borne by the household. This said, only two articles estimated the cost of disabilities due to illness beyond the facility visit [31, 39].
Source of data As a good practice, authors should disclose the source of the data and how they combined data from different sources to generate cost estimates [4], particularly when complementing their data with estimates from secondary sources like WHO-CHOICE, as seen in Sinha et al. [26]. Furthermore, when adopting a retrospective approach, researchers should use as much as possible publicly available datasets to allow other researchers to replicate the research process [4]. Framed as part of a workshop or produced through a co-creative process, accessible inputs would find use in local policymaking and budget planning [41].
4.1 Applications for Cost Data: Economic Burden of Illness
Estimating the economic burden of illness on households through catastrophic health expenditures is an official sustainable development goal indicator for monitoring the progress of financial protection provided by the universal health coverage [46]. Given the importance of economic burden measures for the global health agenda, we examined the studies selected for this systematic review to determine whether they took the additional step to measure the economic burden of illness on bearers of the costs. While 18 studies (60%) contain some measures of the economic burden of illness, 15 studies reported household income or expenditure as the denominator. Only eight of 15 studies provided estimates from primary data collection.
Beyond simply comparing the numerator against the denominator, several studies contain in-depth analyses of the difference in out-of-pocket household expenditure across income quintiles. Ngabo et al. [17] demonstrated the disproportionate impact of the pediatric inpatient admission for GE on the lowest quintile (110%) compared to the highest quintile (21%) in Rwanda [17]. Similarly, Loganathan et al. [32] found that out-of-pocket expenditure of the lowest quintile as a proportion of monthly household income (23.2%) was higher than that of the highest income quintile in Malaysia (5.7%). They also demonstrated that out-of-pocket costs were concentrated among the wealthy [32] based on poverty headcounts.
Furthermore, Burke et al. [18] developed a logistic regression model and identified potential predictors of catastrophic costs such as outpatient status, care seeking at a private hospital, whether treatment was previously sought for the GE episode, and the number of days of experiencing GE before the current visit.
These findings show that additional data on the distribution of income will provide further insights into the economic burden of illness across quintiles and the implications for increasing equity across population subgroups. Future COI studies will benefit from incorporating economic burden-related variables into primary data collection, which will help improve the application of COI estimates to health sector priority setting and budget planning as well as to the advancement of universal health coverage.
4.2 Limitations of this Systematic Review
Considering their value for health technology assessment and program evaluation, the economic burden of illness is likely more frequently assessed than the literature suggests and with varying rigor, as our review suggests. It is not surprising to find COI studies integrated into randomized controlled trials and cost-effectiveness analyses. With our choice of keywords, we chose to capture a significant amount of studies, most of them irrelevant to COI, for the chance to find those hiding any assessments of the COI in their analysis. Notably, we cannot exclude human error from such a long screening process, and we may have missed some articles. Existing systematic reviews helped in limiting this risk [47]. It is possible that language played a role in limiting the search results, especially for articles published in Russian, Japanese, and Chinese: in our selection, one study was published in a regional journal and was not translated to English [48]. This said, with the exclusion of the gray literature and with the coverage of English, French, Spanish, and Portuguese, we believed that we were able to effectively limit this risk.
Another limitation may come from bringing the reported costs to a comparable currency (2018 US$). The inflation correction and the currency conversion may be distorting the COI estimates, particularly those from older articles. Open access to the dataset with raw and corrected data can allow researchers to change the corrections or use the original estimates [9].
Finally, we conducted a brief scoping review of the literature from January 2017 to May 2020 and found several articles that may have been eligible for inclusion post-2016. Particularly, we found two studies assessing the cost of measles in the Federated States of Micronesia [49] and the cost of measles and rubella in Romania [50] — a first for measles and rubella in LMIC. Other studies examined further the cost of GE [51,52,53,54,55,56,57,58,59], pneumonia [51, 52, 59,60,61,62,63], and influenza [64, 65]. Some of them generated COI estimates from a programmatic approach, evaluating the cost of treating diarrhea and pneumonia through an integrated community case management [51, 52, 59]. Such an approach generates questions on the representativeness of the COI estimates, particularly for regions where integrated community case management is not implemented, where alternative care processes are in place, or where it faces logistical shortcomings, such as medication stock-outs, that affect the COI and how people decide to procure healthcare [66].
4.3 Implications for Policy and Research Priority Setting
Our review demonstrates that VPDs represent a significant economic burden both to households and the healthcare system. As COI data become more standardized and are more readily available for different settings, governments or stakeholder organizations will be able to directly compare the economic and financial burden of illnesses and develop policy targets and priorities accordingly. Within the health sector, decision makers can also apply COI data to better understand the financial realities of service utilization for different illnesses and better target interventions focused on improving the equity of healthcare access and utilization. Outside of the health sector, COI data can be used to understand how targeted investments in health may improve household economic well-being or generate spillover gains to the broader economy by freeing up disposable income for savings and non-health consumption, reducing catastrophic health expenditures, and improving labor force participation and productivity.
Using COI to generate transparent monetary assessments can also assist in the reframing of public health spending as investments that reduce incurred costs, rather than as pure costs. Such a reframing can also help to create parity in both languages and in impacts measured for investments in health vs in other sectors that seek to target directly financial and economic growth. The result may be a more direct discussion of health investments as a component of national strategic planning for economic growth and development more broadly.
5 Conclusions
Data were extracted for 37 COI studies conducted on childhood VPD in LMIC, generating a total of 267 different sets of costs. The methodological heterogeneity across studies limited our ability to aggregate and compare costs. The lack of COI studies in LMIC with primary data collection for measles, hepatitis B, rubella, and YF was not surprising, considering the long-standing existence of vaccines that target these diseases. However, the resurgence of these diseases and the global interest towards eradication should motivate the development of COI estimates to assess the impact of such scenarios on healthcare budgets and households.
Data Availability
This study can be replicated with the dataset available in open access on DataVerse with the identifiers https://doi.org/10.7910/dvn/cb6x8k and at: https://dataverse.harvard.edu/dataverse/dove-coi-sr/.
References
Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. BMJ. 2000;320(7245):1335.
Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327–37. https://doi.org/10.3350/cmh.2014.20.4.327.
World Bank. World Bank country and lending groups. Country classification. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 13 May 2020.
Clabaugh G, Ward MM. Cost-of-illness studies in the United States: a systematic review of methodologies used for direct cost. Value Health. 2008;11(1):13–21. https://doi.org/10.1111/j.1524-4733.2007.00210.x.
Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness Studies. Pharmacoeconomics. 2006;24(9):869–90. https://doi.org/10.2165/00019053-200624090-00005.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Hodgson TA, Meiners MR. Cost-of-illness methodology: a guide to current practices and procedures. Milbank Mem Fund Q Health Soc. 1982;60(3):429–62. https://doi.org/10.2307/3349801.
Vassall A, Sweeney S, Kahn J, Gomez GB, Bollinger L, Marseille, et al. Reference case for estimating the costs of global health services and interventions. Project Report. Global Health Cost Consortium; 2017. https://researchonline.lshtm.ac.uk/id/eprint/4653001 Accessed 17 July 2020.
de Broucker G, Sim SY, Brenzel L, Gross M, Patenaude B, Constenla D. Replication data for: the cost of nine pediatric infectious illnesses in low- and middle-income countries: a systematic review of cost of illness studies. V2 ed. Baltimore: Harvard Dataverse; 2020.
World Bank. Official exchange rate (LCU per US$, period average). International Monetary Fund, International Financial Statistics. 2019. https://data.worldbank.org/indicator/PA.NUS.FCRF. Accessed 23 Sept 2019.
World Bank. Consumer price index. International Monetary Fund, International Financial Statistics. 2019. https://data.worldbank.org/indicator/FP.CPI.TOTL. Accessed 23 Sept 2019.
Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3.
Gavi. Annual progress report. Geneva: Gavi, The Vaccine Alliance; 2018.
Bar-Zeev N, Tate JE, Pecenka C, Chikafa J, Mvula H, Wachepa R, et al. Cost-effectiveness of monovalent rotavirus vaccination of infants in Malawi: a postintroduction analysis using individual patient-level costing data. Clin Infect Dis. 2016;62:S220–8. https://doi.org/10.1093/cid/civ1025.
Griffiths MJ, Lemon JV, Rayamajhi A, Poudel P, Shrestha P, Srivastav V, et al. The functional, social and economic impact of acute encephalitis syndrome in Nepal: a longitudinal follow-up study. PLoS Neglect Trop Dis. 2013;7(9):e2383. https://doi.org/10.1371/journal.pntd.0002383.
Jacob J, Joseph TK, Srinivasan R, Kompithra RZ, Simon A, Kang G. Direct and indirect costs of pediatric gastroenteritis in Vellore. India. Indian Pediatr. 2016;53(7):642–4. https://doi.org/10.1007/s13312-016-0903-3.
Ngabo F, Mvundura M, Gazley L, Gatera M, Rugambwa C, Kayonga E, et al. The economic burden attributable to a child’s inpatient admission for diarrheal disease in Rwanda. PLoS One. 2016;11(2):e0149805. https://doi.org/10.1371/journal.pone.0149805.
Burke RM, Smith ER, Dahl RM, Rebolledo PA, Calderón MEC, Cañipa, et al. The economic burden of pediatric gastroenteritis to Bolivian families: a cross-sectional study of correlates of catastrophic cost and overall cost burden. BMC Public Health. 2014;14:642. https://doi.org/10.1186/1471-2458-14-642.
Burke RM, Rebolledo PA, Embrey SR, Wagner LD, Cowden CL, Kelly FM, et al. The burden of pediatric diarrhea: a cross-sectional study of incurred costs and perceptions of cost among Bolivian families. BMC Public Health. 2013;13:708.
Tate JE, Chitambar S, Esposito DH, Sarkar R, Gladstone B, Ramani S, et al. Disease and economic burden of rotavirus diarrhoea in India. Vaccine. 2009;27(Suppl. 5):F18–24. https://doi.org/10.1016/j.vaccine.2009.08.098.
Flem ET, Latipov R, Nurmatov ZS, Xue Y, Kasymbekova KT, Rheingans RD. Costs of diarrheal disease and the cost-effectiveness of a rotavirus vaccination program in Kyrgyzstan. J Infect Dis. 2009;200(Suppl. 1):S195–202. https://doi.org/10.1086/605040.
Wilopo SA, Kilgore P, Kosen S, Soenarto Y, Aminah S, Cahyono, et al. Economic evaluation of a routine rotavirus vaccination programme in Indonesia. Vaccine. 2009;27(Suppl. 5):F67–74. https://doi.org/10.1016/j.vaccine.2009.09.040.
Chola L, Robberstad B. Estimating average inpatient and outpatient costs and childhood pneumonia and diarrhoea treatment costs in an urban health centre in Zambia. Cost Eff Resour Alloc. 2009;7:16. https://doi.org/10.1186/1478-7547-7-16.
MacIntyre UE, De Villiers FPR. The economic burden of diarrheal disease in a tertiary level hospital, Gauteng. South Africa. Journal of Infectious Diseases. 2010;202(Suppl. 1):S116–25. https://doi.org/10.1086/653560.
Anh DD, Riewpaiboon A, Tho H, Kim SA, Nyambat B, Kilgore P. Treatment costs of pneumonia, meningitis, sepsis, and other diseases among hospitalized children in Viet Nam. J Health Popul Nutr. 2010;28(5):436–42.
Sinha A, Kim S, Ginsberg G, Franklin H, Kohberger R, Strutton D, et al. Economic burden of acute lower respiratory tract infection in South African children. Paediatr Int Child Health. 2012;32(2):65–73. https://doi.org/10.1179/2046905512Y.0000000010.
Temple B, Griffiths UK, Mulholland EK, Ratu FT, Tikoduadua L, Russell FM. The cost of outpatient pneumonia in children < 5 years of age in Fiji. Trop Med Int Health. 2012;17(2):197–203. https://doi.org/10.1111/j.1365-3156.2011.02897.x.
Lee WS, Poo MI, Nagaraj S. Estimates of economic burden of providing inpatient care in childhood rotavirus gastroenteritis from Malaysia. J Paediatr Child Health. 2007;43(12):818–25. https://doi.org/10.1111/j.1440-1754.2007.01160.x.
Hussain H, Waters H, Omer SB, Khan A, Baig IY, Mistry R, et al. The cost of treatment for child pneumonias and meningitis in the Northern Areas of Pakistan. Int J Health Plann Manage. 2006;21(3):229–38.
Chai PF, Lee WS. Out-of-pocket costs associated with rotavirus gastroenteritis requiring hospitalization in Malaysia. Vaccine. 2009;27(Suppl. 5):F112–5. https://doi.org/10.1016/j.vaccine.2009.08.069.
Platonov AE, Griffiths UK, Voeykova MV, Platonova OV, Shakhanina IL, Chistyakova GG, et al. Economic evaluation of Haemophilus influenzae type b vaccination in Moscow. Russian Federation. Vaccine. 2006;24(13):2367–76. https://doi.org/10.1016/j.vaccine.2005.11.054.
Loganathan T, Lee WS, Lee KF, Jit M, Ng CW. Household catastrophic healthcare expenditure and impoverishment due to rotavirus gastroenteritis requiring hospitalization in Malaysia. PLoS One. 2015;10(5):e0125878. https://doi.org/10.1371/journal.pone.0125878.
Tate JE, Rheingans RD, Oreilly CE, Obonyo B, Burton DC, Tornheim JA, et al. Rotavirus disease burden and impact and cost- effectiveness of a rotavirus vaccination program in Kenya. J Infect Dis. 2009;200(Suppl. 1):S76–84. https://doi.org/10.1086/605058.
Madsen HO, Hanehøj M, Das AR, Moses PD, Rose W, Puliyel M, et al. Costing of severe pneumonia in hospitalized infants and children aged 2-36 months, at a secondary and tertiary level hospital of a not-for-profit organization. Trop Med Int Health. 2009;14(10):1315–22. https://doi.org/10.1111/j.1365-3156.2009.02374.x.
Hussain H, Waters H, Khan AJ, Omer SB, Halsey NA. Economic analysis of childhood pneumonia in Northern Pakistan. Health Policy Plan. 2008;23(6):438–42. https://doi.org/10.1093/heapol/czn033.
Fischer TK, Anh DD, Antil L, Cat NDL, Kilgore PE, Thiem VD, et al. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192(10):1720–6. https://doi.org/10.1086/497339.
Le P, Griffiths UK, Anh DD, Franzini L, Chan W, Pham H, et al. The economic burden of pneumonia and meningitis among children less than five years old in Hanoi. Vietnam. Trop Med Int Health. 2014;19(11):1321–7. https://doi.org/10.1111/tmi.12370.
Alkoshi S, Leshem E, Parashar UD, Dahlui M. Anticipating rotavirus vaccines: a pre-vaccine assessment of incidence and economic burden of rotavirus hospitalizations among children < 5 year of age in Libya, 2012-13. BMC Public Health. 2015;15:26. https://doi.org/10.1186/s12889-015-1400-7.
Usuf E, Mackenzie G, Sambou S, Atherly D, Suraratdecha C. The economic burden of childhood pneumococcal diseases in The Gambia. Cost Eff Resour Alloc. 2016;14:4. https://doi.org/10.1186/s12962-016-0053-4.
Patel AB, Bang A, Singh M, Dhande L, Chelliah LR, Malik A, et al. A randomized controlled trial of hospital versus home based therapy with oral amoxicillin for severe pneumonia in children aged 3–59 months: the IndiaCLEN Severe Pneumonia Oral Therapy (ISPOT) Study. BMC Pediatr. 2015;15:186. https://doi.org/10.1186/s12887-015-0510-9.
Rice DP. Estimating the cost of illness. Am J Public Health Nat Health. 1967;57(3):424–40. https://doi.org/10.2105/ajph.57.3.424.
Ashraf H, Mahmud R, Alam NH, Jahan SA, Kamal SM, Haque F, et al. Randomized controlled trial of day care versus hospital care of severe pneumonia in Bangladesh. Pediatrics. 2010;126(4):e807–15. https://doi.org/10.1542/peds.2009-3631.
Ayieko P, Irimu G, Ogero M, Mwaniki P, Malla L, Julius T, et al. Effect of enhancing audit and feedback on uptake of childhood pneumonia treatment policy in hospitals that are part of a clinical network: a cluster randomized trial. Implement Sci. 2019;14(1):20. https://doi.org/10.1186/s13012-019-0868-4.
Mathew A, Srinivasan R, Venugopal S, Kang G. Direct medical costs in children with rotavirus and non-rotavirus diarrhea admitted to a pediatric intensive care unit and high dependency unit in Delhi. Indian Pediatr. 2016;53(7):639–41.
Kitchin OP, Wessels F, Masekela R, Becker P, Green RJ. Costs of admission for paediatric pneumonia in a setting of human immunodeficiency virus infection. Int J tuberculosis Lung Dis. 2011;15(12):1702–7. https://doi.org/10.5588/ijtld.11.0167.
World Health Organization; International Bank for Reconstruction and Development/The World Bank. Tracking universal health coverage: 2017 global monitoring report. Geneva: World Health Organization; 2017.
Zhang S, Sammon PM, King I, Andrade AL, Toscano CM, Araujo SN, et al. Cost of management of severe pneumonia in young children: systematic analysis. J Glob Health. 2016;6(1):010408. https://doi.org/10.7189/jogh.06.010408.
Guzmán NA, De La Hoz Restrepo F, Higuera AB, Pastor D, Di Fabio JL. The economic costs of pneumonia in children under 2 years of age in Colombia. Rev Panam Salud Publica. 2005;17(3):178–83.
Pike J, Tippins A, Nyaku M, Eckert M, Helgenberger L, Underwood JM. Cost of a measles outbreak in a remote island economy: 2014 Federated States of Micronesia measles outbreak. Vaccine. 2017;35(43):5905–11. https://doi.org/10.1016/j.vaccine.2017.08.075.
Njau J, Janta D, Stanescu A, Pallas SS, Pistol A, Khetsuriani N, et al. Assessment of economic burden of concurrent measles and rubella outbreaks, Romania, 2011-2012. Emerg Infect Dis. 2019;25(6):1101–9. https://doi.org/10.3201/eid2506.180339.
Daviaud E, Besada D, Leon N, Rohde S, Sanders D, Oliphant N, et al. Costs of implementing integrated community case management (iCCM) in six African countries: implications for sustainability. J Glob Health. 2017;7(1):010403. https://doi.org/10.7189/jogh.07.010403.
Escribano Ferrer B, Hansen KS, Gyapong M, Bruce J, Narh Bana SA, Narh CT, et al. Cost-effectiveness analysis of the national implementation of integrated community case management and community-based health planning and services in Ghana for the treatment of malaria, diarrhoea and pneumonia. Malar J. 2017;16(1):277. https://doi.org/10.1186/s12936-017-1906-9.
Halder AK, Luby SP, Akhter S, Ghosh PK, Johnston RB, Unicomb L. Incidences and costs of illness for diarrhea and acute respiratory infections for children < 5 years of age in rural Bangladesh. Am J Trop Med Hyg. 2017;96(4):953–60. https://doi.org/10.4269/ajtmh.16-0005.
Hendrix N, Bar-Zeev N, Atherly D, Chikafa J, Mvula H, Wachepa R, et al. The economic impact of childhood acute gastroenteritis on Malawian families and the healthcare system. BMJ Open. 2017;7(9):e017347. https://doi.org/10.1136/bmjopen-2017-017347.
Koksal T, Akelma AZ, Koksal AO, Kutukoglu I, Ozdemir O, Yuksel CN, et al. Cost-effectiveness of rotavirus vaccination in Turkey. J Microbiol Immunol Infect. 2017;50(5):693–9. https://doi.org/10.1016/j.jmii.2016.03.005.
Kukla M, McKay N, Rheingans R, Harman J, Schumacher J, Kotloff KL, et al. The effect of costs on Kenyan households’ demand for medical care: why time and distance matter. Health Policy Plan. 2017;32(10):1397–406. https://doi.org/10.1093/heapol/czx120.
Nonvignon J, Atherly D, Pecenka C, Aikins M, Gazley L, Groman D, et al. Cost-effectiveness of rotavirus vaccination in Ghana: examining impacts from 2012 to 2031. Vaccine. 2018;36(47):7215–21. https://doi.org/10.1016/j.vaccine.2017.11.080.
Rochanathimoke O, Riewpaiboon A, Tharmaphornpilas P, Jiamsiri S, Thavorncharoensap M, Postma MJ. Economic burden of rotavirus diarrhea in Thailand: rfrom a pilot study on rotavirus vaccination. Vaccine. 2019;37(4):587–94. https://doi.org/10.1016/j.vaccine.2018.12.013.
Soremekun S, Kasteng F, Lingam R, Vassall A, Kertho E, Settumba S, et al. Variation in the quality and out-of-pocket cost of treatment for childhood malaria, diarrhoea, and pneumonia: community and facility based care in rural Uganda. PLoS One. 2018;13(11):e0200543. https://doi.org/10.1371/journal.pone.0200543.
Andrade AL, Afonso ET, Minamisava R, Bierrenbach AL, Cristo EB, Morais-Neto OL, et al. Direct and indirect impact of 10-valent pneumococcal conjugate vaccine introduction on pneumonia hospitalizations and economic burden in all age-groups in Brazil: a time-series analysis. PLoS One. 2017;12(9):e0184204. https://doi.org/10.1371/journal.pone.0184204.
Ezoji K, Yaghoubi M, Nojomi M, Mahmoody S, Zahraie SM, Moradi-Lakeh M, et al. Cost-effectiveness of introducing the pneumococcal conjugate vaccine for children under 5 years in the Islamic Republic of Iran. East Mediterr Health J. 2019;25(10):686–97. https://doi.org/10.26719/emhj.19.039.
Kebede TT, Svensson M, Addissie A, Trollfors B, Andersson R. Cost-effectiveness of childhood pneumococcal vaccination program in Ethiopia: results from a quasi-experimental evaluation. BMC Public Health. 2019;19(1):1078. https://doi.org/10.1186/s12889-019-7423-8.
Shen K, Wasserman M, Liu D, Yang YH, Yang J, Guzauskas GF, et al. Estimating the cost-effectiveness of an infant 13-valent pneumococcal conjugate vaccine national immunization program in China. PLoS One. 2018;13(7):e0201245. https://doi.org/10.1371/journal.pone.0201245.
Orenstein EW, Orenstein LA, Diarra K, Djiteye M, Sidibe D, Haidara FC, et al. Cost-effectiveness of maternal influenza immunization in Bamako, Mali: a decision analysis. PLoS One. 2017;12(2):e0171499. https://doi.org/10.1371/journal.pone.0171499.
Kittikraisak W, Suntarattiwong P, Kanjanapattanakul W, Ditsungnoen D, Klungthong C, Lindblade KA, et al. Comparison of incidence and cost of influenza between healthy and high-risk children < 60 months old in Thailand, 2011-2015. PLoS One. 2018;13(5):e0197207. https://doi.org/10.1371/journal.pone.0197207.
Nanyonjo A, Counihan H, Siduda SG, Belay K, Sebikaari G, Tibenderana J. Institutionalization of integrated community case management into national health systems in low- and middle-income countries: a scoping review of the literature. Glob Health Action. 2019;12(1):1678283. https://doi.org/10.1080/16549716.2019.1678283.
Soltani MS, Ben Salah A, Bouanene I, Trabelsi A, Sfar MT, Harbi A, et al. Epidemiology and medical cost of hospitalization due to rotavirus gastroenteritis among children under 5 years of age in the central-east of Tunisia. Eastern Med Health J. 2015;21(8):584–90.
Zhou L, Situ S, Huang T, Hu S, Wang X, Zhu, et al. Direct medical cost of influenza-related hospitalizations among severe acute respiratory infections cases in three provinces in China. PLoS One. 2013;8(5):e63788. https://doi.org/10.1371/journal.pone.0063788.
Constenla D. Evaluating the costs of pneumococcal disease in selected Latin American countries. Rev Panam Salud Publica. 2007;22(4):268–78.
Riewpaiboon A, Shin S, Le TP, Vu DT, Nguyen TH, Alexander N, et al. Cost of rotavirus diarrhea for programmatic evaluation of vaccination in Vietnam. BMC Public Health. 2016;16(1):777. https://doi.org/10.1186/s12889-016-3458-2.
Sadruddin S, Shehzad S, Bari A, Khan A, Ibad Ul H, Khan A, et al. Household costs for treatment of severe pneumonia in Pakistan. Am J Trop Med Hyg. 2012;87(Suppl. 5):137–43. https://doi.org/10.4269/ajtmh.2012.12-0242.
Ayieko P, Akumu AO, Griffiths UK, English M. The economic burden of inpatient paediatric care in Kenya: household and provider costs for treatment of pneumonia, malaria and meningitis. Cost Eff Resour Alloc. 2009;7:3. https://doi.org/10.1186/1478-7547-7-3.
Acknowledgements
We acknowledge all contributors to this work. Margaret Gross, Jorge Martin del Campo, Jenny Lee, and Gatien de Broucker performed the screening process for 12,792 articles. Diane Coraggio, Grace Morgan, Portia Pan, Alie Tawah, Karuna Luthra, Jeong Ah Cho, So Yeon Kang, So Yoon Sim, and Gatien de Broucker all contributed to the design and testing of the data extraction tool and contributed to data extraction. Gatien de Broucker, So Yoon Sim, Margaret Gross, Dagna Constenla, Bryan Patenaude, and Logan Brenzel analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Contributions
GB and MG performed the screening process. GB and SYS extracted the data from the articles and developed the database. GB and SYS analyzed the data. GB, SYS, MG, DC, BP, and LB wrote the manuscript.
Corresponding author
Ethics declarations
Funding
This work was funded by the Bill & Melinda Gates Foundation as part of the Decade of Vaccine Economics (DOVE) project.
Conflict of Interest
Dagna Constenla joined GSK as an employee while the manuscript was being reviewed and once the analysis of the paper had already been completed. Logan Brenzel works for the Bill & Melinda Gates Foundation.
Code Availability
The dataset is formatted in MS Excel 2013 (XLSX file) that can be opened in any open source spreadsheet software.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Broucker, G., Sim, S.Y., Brenzel, L. et al. Cost of Nine Pediatric Infectious Illnesses in Low- and Middle-Income Countries: A Systematic Review of Cost-of-Illness Studies. PharmacoEconomics 38, 1071–1094 (2020). https://doi.org/10.1007/s40273-020-00940-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-020-00940-4