Abstract
Objective
The objective of this study was to gain insights into the attitudes of men with lower urinary tract symptoms towards deprescribing alpha-blockers and to assess their willingness to participate in a planned discontinuation trial.
Methods
This was a cross-sectional questionnaire study. Men aged 30 years and older with lower urinary tract symptoms, who were first prescribed an alpha-blocker in 2015 or 2016, were selected from a population-based prescription database. We recorded lower urinary tract symptom severity (e.g., International Prostate Symptom Score and Overactive Bladder questionnaire) and patient characteristics (e.g., comorbidity and polypharmacy). The linguistically validated Dutch version of the revised Patients’ Attitudes Towards Deprescribing (rPATD) questionnaire was also used, to which we added ten specific questions on attitudes towards the deprescribing of alpha-blockers. Information about a future discontinuation trial on alpha-blockers was then provided and participants were asked to indicate if they would participate. We explored the explanatory factors for the willingness to participate by logistic regression analyses.
Results
Of the 1380 patients in the database, 421 were using an alpha-blocker, and 195 completed the questionnaire. Of these, 16 men were excluded because of indwelling catheter use or unknown indication. The mean age of the 179 participants was 69.4 (standard deviation 9.2) years. Most men were satisfied with their current therapy, but almost all (93%) were willing to stop the medicine at the request of a doctor. Therefore, most men (61%) were willing to participate in the proposed alpha-blocker discontinuation trial. Willingness to stop therapy was affected by patients’ perceptions of the appropriateness of alpha-blocker therapy and concerns about stopping that therapy.
Conclusions
Although men who use alpha-blockers are generally satisfied with their current therapy, most will participate in a discontinuation trial.
Similar content being viewed by others
This is the first study to have used the linguistically validated Dutch version of the revised Patients’ Attitudes Towards Deprescribing Questionnaire (rPATD). |
Patients appear to be unconcerned about stopping alpha-blocker therapy and most are willing to stop their treatment at the suggestion of a doctor. |
Most alpha-blocker users would be willing to participate in a discontinuation trial if they were invited and patients’ perceptions of the appropriateness of alpha-blocker therapy, as well as their concerns about stopping that treatment, are important to this willingness. |
1 Introduction
Alpha-blockers are the cornerstone of drug treatment for male lower urinary tract symptoms (LUTS) [1]. The prevalence of moderate-to-severe LUTS varies between 20 and 25% and it increases with age [2]. In the Netherlands, half of men visiting a general practitioner (GP) with LUTS receive drug therapy. Over 80% of these men receive alpha-blocker therapy, accounting for approximately 270,000 male users at a cost of 15 million euros in 2016 [3, 4]. Alpha-blockers aim to reduce the muscle tone of the prostate and the bladder output resistance by blocking the alpha-1-receptors [5]. The major effect of alpha-blockers is measurable within 2 weeks. Little is known about the clinical value of the alpha-blocker and the duration of prescription. A systematic literature review and meta-analysis, performed by the National Institute for Health and Clinical Excellence, revealed a modest decrease in symptom severity of limited clinical relevance [6]. Prolonged use of alpha-blockers is expensive and may be associated with avoidable adverse effects, such as postural hypotension, headache, dizziness, fatigue, and ejaculation disorder. It is unclear if adverse-effect profiles differ between ages, although especially postural hypotension may be more prevalent with increasing age.
The Dutch guideline for GPs, issued for the assessment and treatment of LUTS in adult men (aged 18 years and older), recommends discontinuing alpha-blockers 3–6 months after symptom relief from LUTS; however, it presented no evidence to support this advice [2]. By contrast, it is notable that the national guideline for Dutch urologists offers no advice about discontinuation, and that the European Association of Urology only recommends considering discontinuation when alpha-blockers are used in combination therapy with 5-alpha reductase inhibitors [1, 5]. As has been investigated in a Dutch pharmacy-based study, the discontinuation rate 1 year after the initiation of treatment was 60.3–66.1%, without relevant differences between prescriptions from urologists and GPs [7]. Discontinuation of the alpha-blocker is important in respect of the unknown long-term clinical value, the avoidable side effects, and healthcare costs. Although some studies reported on the clinical outcome of discontinuation of alpha-blockers, no information is available on the attitudes of patients [8, 9].
Discontinuation is appropriate when drugs are ineffective, unnecessary, or present a high risk, especially in older patients with multi-morbidity who are at risk of polypharmacy. The barriers and enablers in so-called deprescribing, the process of discontinuing an inappropriate drug under the supervision of a healthcare professional, can be studied using the recently validated version of the revised Patients’ Attitudes Towards Deprescribing (rPATD) questionnaire [10, 11].
The purpose of this cross-sectional questionnaire study was to gain insights into the attitudes of men aged 30 years and over with LUTS towards deprescribing alpha-blocker therapy. We also wanted to assess the willingness of those men to participate in a planned discontinuation trial of alpha-blockers and to analyse what factors influenced that willingness to participate.
2 Methods
2.1 Study Design and Population
We conducted a cross-sectional questionnaire survey among men with LUTS who were receiving alpha-blocker therapy. Men were selected from 20 pharmacies collaborating with the University of Groningen pharmacy prescription database (IADB.nl). This is a growing database that contains prescription data for more than 20 years from approximately 60 community pharmacies, covering an estimated 600,000 patients. Registration in the database is irrespective of healthcare insurance, age, or sex. Prescription rates in the database have been shown to be representative of the Netherlands as a whole, and the database is widely used for research. Each person is individually tracked throughout the database period and prescription records contain information on the date of dispensing, the quantity dispensed, the dose regimen, the number of days the prescription is valid, the prescribing physician, and the Anatomical Therapeutic Chemical code. Each patient has a unique anonymous identifier; date of birth and sex are known. Because of the high patient-pharmacy commitment in the Netherlands, the medication records for each patient are virtually complete, except for over-the-counter drugs and medication dispensed during hospitalization [12].
We selected patients based on the following criteria: men aged at least 30 years; a first alpha-blocker prescription in the years 2015 or 2016, prescribed as monotherapy (e.g., no combination therapy with 5-alpha reductase inhibitors), with an Anatomical Therapeutic Chemical Classification code G04CA01–04 (Alfuzosine, Tamsulosine, Terazosine and Silodosine) [4], and no prescriptions for these codes in the 2 preceding years; and continued use of the alpha-blocker to the day of invitation. This age group was chosen as alpha-blockers are prescribed in these age groups. Invitations were sent from March until September 2017, thus the minimum duration of participants taking an alpha-blocker was at least 3 months. We excluded men who were using the alpha-blocker with an indwelling catheter and for whom we could not determine the indication.
Invitations were sent on behalf of the participating pharmacies, using a patient identification code, from the IADB.nl database. Thereafter, we used encrypted respondent numbers on the invitations to enable the sending of a reminder after 2 weeks in case of non-response. Questionnaires could be completed on paper or online. The Medical Ethics Committee of the University Medical Centre Groningen gave approval to conduct this study (METc2017/008). In lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
2.2 Data Collection
We recorded the age of each participant and asked them to complete four questionnaires: the general and the alpha-blocker-specific rPATD questionnaires, the International Prostate Symptom Index (IPSS) [13], and the Overactive Bladder questionnaire (OAB-q) [14]. The IPSS is the main questionnaire on the severity of LUTS, used in the field of urology. It consists of seven questions on different symptoms. Sum scores range from zero to 35 points, and we categorized scores as mild (1–7 points), moderate (8–19), or severe (20–35). The OAB-q is a questionnaire that assesses symptom bother and health-related quality of life. This questionnaire consists of six questions, and scores range from zero to 100. We presented the mean score for this questionnaire as no cut-off values are available to categorize symptom severity. We also asked why the alpha-blocker had been prescribed (i.e., LUTS, indwelling catheter, or unknown indication), the first prescriber of the alpha-blocker (i.e., urologist or GP), how many medications were currently prescribed and about any diagnosed comorbidities (e.g., cardiovascular disease, diabetes mellitus, chronic obstructive pulmonary disease, prostate or bladder surgery, and prostate or bladder cancer). Finally, to assess willingness to participate in a planned discontinuation trial of alpha-blockers, we provided information about the trial in written and video formats. Men were subsequently asked to indicate their willingness to participate in such a study if invited (yes or no).
2.2.1 General and Alpha-Blocker-Specific rPATD Questionnaires
To measure attitudes towards deprescribing, we translated and linguistically validated the rPATD questionnaire, using the World Health Organization guideline ‘Process of translation and adaptation of instruments’ [15]. The translated questionnaire and the translation and validation process are available on request.
The rPATD questionnaire consists of four factors: ‘overall burden’, ‘appropriateness’, ‘concerns about stopping’, and ‘involvement’ [11]. Each factor contains five questions or statements (see the Electronic Supplementary Material for details). In addition, two general questions are asked: ‘Overall, I am satisfied with my current medicines’ (Question A) and ‘If my doctor said it was possible, I would be willing to stop one or more of my regular medicines’ (Question B). All responses were scored on 5-point Likert scales, ranging from strongly disagree (1) to strongly agree (5). The rPATD was originally developed to collect information on attitudes towards deprescribing of drugs in general. Therefore, to investigate attitudes towards alpha-blocker deprescribing, the questions in the ‘appropriateness’ and ‘concerns about stopping’ factors were amended by changing the word ‘medicines’ to ‘alpha-blocker’ to create alpha-blocker-specific rPATD factors. All participants were asked to answer the questions about attitudes towards alpha-blocker deprescribing, but we requested only participants with three or more concomitant drugs to complete the questions about deprescribing in general. We felt that deprescribing is especially important in the case of polypharmacy; we decided not to bother participants with two drugs or fewer with the completion of the rPATD.
2.3 Analyses
All statistical analyses were performed with IBM SPSS Version 23.0 (IBM Corp., Armonk, NY, USA) and all p values ≤ 0.05 were considered statistically significant. We compared responders to non-responders with respect to age and the number of prescribed medications. Mean ages were compared by the independent t-test, and the median numbers (interquartile range [IQR]) of prescribed medications were compared using the Mann–Whitney U test. In the main analyses, we assessed the attitudes towards alpha-blocker deprescribing and the percentage of men who would be willing to participate in a discontinuation trial.
For the attitudes towards deprescribing, we estimated the mean score for each rPATD factor (two alpha-blocker-specific factors and four general factors, range 1–5) [11]. The questions of the appropriateness factors were scored inversely, with a higher score indicating that the medication was considered appropriate. In lieu of any specific guidelines for interpreting the factor scores at the time of analysis, we categorized each alpha-blocker-specific rPATD factor and each general rPATD factor into ‘disagree’ (mean factor score, 1.0–2.4), ‘neutral’ (2.5–3.5), and ‘agree’ (3.6–5.0) to describe the attitudes. The results were then expressed as percentages.
Finally, we calculated the percentage of participants taking alpha-blockers who would be willing to participate in a discontinuation trial. To analyse the explanatory factors for this willingness, we performed univariate logistic regression analyses, entering the IPSS, IPSS-quality of life (QoL), OAB-q, alpha-blocker-specific rPATD factors, general rPATD factors, age, and number of currently prescribed medications. The odds ratios (ORs) and corresponding 95% confidence interval (CIs) are presented.
3 Results
3.1 Participants
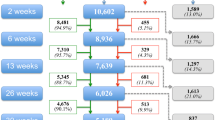
We identified 1380 patients through the IADB.nl database, of whom 421 were still using an alpha-blocker on the day of invitation and 195 (46.3%) completed the questionnaire. We excluded 16 men because of indwelling catheter use or unknown indication, making 179 participants the basis of our research. The study flow is shown in Fig. 1, and the characteristics of the participants are summarized in Table 1.
The mean age of responders was 69.4 (standard deviation 9.2) years, compared with 71.8 (standard deviation 10.2) years in non-responders (independent t test, p = 0.01). 86% was older than 60 years of age. The median number of self-reported drugs was 4.0 (IQR 2.0–6.0) and 41.6% of patients were prescribed more than five drugs. The number of drugs did not differ between responders and non-responders (Mann–Whitney U test, p = 0.08). Many participants also reported one or more chronic diseases (37.2%), with cardiovascular disease mentioned most frequently. The mean IPSS score was 14.4 (standard deviation 6.7), with moderate or severe LUTS reported in 57.1% and 26.3% of patients, respectively. The median IPSS-QoL and OAB-q scores were 3.0 (IQR 2.0–4.0) and 30.0 (IQR 16.7–43.3), respectively. Most first prescriptions of alpha-blockers were provided by a GP (65.7%).
3.2 Attitudes Towards Deprescribing
The attitudes towards deprescribing are shown in Fig. 2. Most participants reported being satisfied with their current drugs (79.2%), but they were still willing to stop one or more of them if their doctor indicated that it was possible (92.7%). A small group of participants (12.1%) reported that their medications were a burden, and most (91.3%) were highly involved with their medications. Regarding appropriateness, 34.7% considered their general medications to be appropriate and 43.8% considered their alpha-blocker therapy to be appropriate. Only 5.6% of all participants reported concerns about stopping a drug and 7.3% reported concerns about stopping the alpha-blocker.
3.3 Willingness to Participate in a Discontinuation Trial
About two thirds of the alpha-blocker users (61.2%) were willing to participate in a discontinuation trial. This willingness was lower when patients considered the alpha-blocker to be an appropriate drug (OR 0.47, 95% CI 0.28–0.78) or when men reported more concerns about stopping the alpha-blocker (OR 0.53, 95% CI 0.32–0.87), as shown in Table 2. Willingness to participate was higher in men who would be willing to stop one or more of their regular medicines at the suggestion of their doctor (OR 1.90, 95% CI 1.06–3.42), and in men with higher IPSS-QoL scores (OR 1.23, 95% CI 1.00–1.51). Ultimately, willingness to participate was independent of LUTS severity, as assessed by the IPSS and OAB-q scores.
4 Discussion
In this study, we assessed the attitudes of 179 male patients with LUTS towards alpha-blocker deprescribing, using the recently revised rPATD. Notably, most users were willing to stop therapy at the request of a doctor and were prepared to participate in a discontinuation trial for alpha-blockers. To the best of our knowledge, this is the first study to have looked into this topic.
This study has some clear limitations. First, we only applied a questionnaire to collect data. The strength of this questionnaire is that the main part consisted of separate thoroughly validated questionnaires. For the rPATD translation, we have followed the guidelines for linguistic validation of questionnaires. The response rate in our study hampers firm conclusions to be drawn, as selection bias may be present. Previous research on deprescribing especially focused on older age groups (65 years and older) [16, 17]. We aimed to include younger patients, as for this group deprescribing may also be highly relevant. As the majority of men who receive alpha-blockers are aged older than 60 years, the group of younger men remained small. However, we believe that this first study in this field provides important insights in this topic. Future research should include qualitative methods, such as interviews or focus groups. In addition, the questions about the willingness to stop medication and to participate in the discontinuation trial are hypothetical, thus further research in clinical practice is needed. Additional validation of the alpha-blocker-specific rPATD is also needed in future research.
In this study, four in five participants were satisfied with their current drugs but the majority of men (92.7%) were willing to stop one or more drugs when their doctor would indicate that this was possible. These findings are in line with recent previous studies, focussing on older age groups [11, 16, 17]. No previous studies were performed on the discontinuation of any urological treatment. We found that almost half of all participants in the present study considered their alpha-blocker therapy to be appropriate, which was larger than the appropriateness scale on other drugs. Only a minority of alpha-blocker users had concerns about stopping. In addition, most of the alpha-blocker users reported that they were satisfied with their current drugs, yet almost all would be willing to stop a medicine if a doctor told them it was possible (93%).
If invited, about two thirds of our participants indicated that they would take part in a discontinuation trial. Predictive factors for participating in such a trial were ‘considering the alpha-blocker an appropriate medicine’, ‘having more concerns about stopping the alpha-blocker’, and agreeing with the statement ‘If my doctor said it was possible, I would be willing to stop one or more of my regular medicines’. This is in line with previous research in Australia in which the willingness to stop a medication is dependent on the appropriateness of a medicine and the concerns of stopping it [17]. In addition, willingness to discontinue therapy was not associated with LUTS severity, though there was a tendency for men who were less satisfied with their LUTS (as assessed by the LUTS-QoL) to be more willing to participate. We felt this finding was counterintuitive, especially given our clear explanation to participants that the medication would be stopped in any discontinuation trial. A possible explanation for this finding is that patients who are dissatisfied may be more willing to try anything different, even discontinuation of the current therapy. Qualitative research may help to clarify the issues underlying this finding.
Reducing drug use is important in modern practice, especially considering the negative effects of polypharmacy. The rate of polypharmacy in our patients (42%) was slightly higher than the reported rate in the Dutch population aged older than 65 years (i.e., 25–38%) based on pharmacy dispensing [18]. This difference is only small, but it might reflect selection bias in our study. Polypharmacy increases the chance of inappropriate drug prescribing with negative clinical and financial consequences [19]. A lack of data about the long-term effect of alpha-blocker use should prompt clinicians to consider deprescribing whenever possible.
To optimize the deprescribing process, it is important to study the barriers and enablers associated with that process. We added specific questions to study attitudes towards alpha-blocker deprescribing and feel that such an approach could be invaluable both in future research and in daily practice. Indeed, it may be possible to replace the specific drug in the questionnaire to allow research into other therapies; however, before the disease-specific component of the questionnaire is implemented more widely, additional validation will be required in a larger cohort. In future research, it would be interesting to change the general questions (e.g., about satisfaction with medication and willingness to stop at the request of a doctor) to questions about a specific medicine. Another important issue in deprescribing is the impact of health literacy. In our study, we did not include literacy scores, although we attempted to provide all information to participants in an easy-to-use format (both printed materials and supportive video messages).
5 Conclusions
Patients appear to be unconcerned about stopping alpha-blocker therapy and most are willing to stop their treatment at the suggestion of a doctor. In particular, our data indicate that most alpha-blocker users would be willing to participate in a discontinuation trial if they were invited. Patients’ perceptions of the appropriateness of alpha-blocker therapy, as well as their concerns about stopping that treatment, are important to this willingness.
References
Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–109.
Blanker MH, Breed SA, van der Heide W, et al. NHG-standaard mictieklachten bij mannen. Huisarts en Wetenschap. 2013;3:114–22.
Verhamme KM, Dieleman JP, Bleumink GS, et al. Treatment strategies, patterns of drug use and treatment discontinuation in men with LUTS suggestive of benign prostatic hyperplasia: the Triumph project. Eur Urol. 2003;44(5):539–45.
Zorginstituut Nederland. GIPdatabank. https://www.gipdatabank.nl/databank. Accessed Jul 2019.
Nederlandse Vereniging voor Urologie. Diagnostiek en behandeling van LUTS/BPH. http://www.nvu.nl/kwaliteit/richtlijnen.aspx. Accessed Jul 2019.
Jefferies M, Cox A, Bennett A, et al. Management of lower urinary tract symptoms in men. Br J Hosp Med (Lond). 2013;74(9):518–22.
Hordijk IMJ, Steffens MG, Hak E, Blanker MH. Continuation rates of alpha-blockers mono-therapy in adult men, prescribed by urologists or general practitioners: a pharmacy-based study. World J Urol. 2018;37(8):1659–64.
Yokoyama T, Watanabe T, Saika T, et al. Natural course of lower urinary tract symptoms following discontinuation of alpha-1-adrenergic blockers in patients with benign prostatic hyperplasia. Int J Urol. 2007;14(7):598–601.
Chung JH, Lee JY, Kang DH, et al. Evaluation of patient outcome after discontinuation of alfuzosin treatment for benign prostatic hyperplasia: a multicentre, prospective study. Int J Clin Pract. 2013;67(9):870–5.
Reeve E, Gnjidic D, Long J, et al. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254–68.
Reeve E, Low LF, Shakib S, et al. Development and validation of the revised patients’ attitudes towards deprescribing (rPATD) questionnaire: versions for older adults and caregivers. Drugs Aging. 2016;33(12):913–28.
Visser ST, Schuiling-Veninga CC, Bos JH, et al. The population-based prescription database IADB.nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):285–92.
Barry MJ, Fowler FJ, O’Leary MP, Measurement Committee of the American Urological Association, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5):1549–57.
Coyne KS, Matza LS, Thompson CL. The responsiveness of the overactive bladder questionnaire (OAB-q). Qual Life Res. 2005;14(3):849–55.
World Health Organization. Process of translation and adaptation of instruments. http://www.who.int/substance_abuse/research_tools/translation/en/. Accessed Jul 2019.
Reeve E, Wolff JL, Skehan M, et al. Assessment of attitudes toward deprescribing in older medicare beneficiaries in the united states. JAMA Intern Med. 2018;178(12):1673–80.
Reeve E, Low L, Hilmer SN. Attitudes of older adults and caregivers in Australia toward deprescribing. J Am Geriatr Soc. 2019;67(6):1204–10.
Stichting Farmaceutische Kengetallen (SFK). Sterke toename aantal polyfarmacie patiënten. Pharm Weekbl. 2016;151(27/28):11.
Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One. 2012;7(8):e43617.
Acknowledgements
We are grateful to Emily Reeve for the help provided in translating the revised Patients’ Attitudes Towards Deprescribing questionnaire into Dutch. We also thank Dr. Robert Sykes for providing editorial services in the final drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflict of interest
Malou Edelman, Petra Jellema, Eelko Hak, Petra Denig, and Marco H. Blanker have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The Medical Ethics Committee of the University Medical Centre Groningen gave approval to conduct this study (METc2017/008). In lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Edelman, M., Jellema, P., Hak, E. et al. Patients’ Attitudes Towards Deprescribing Alpha-Blockers and Their Willingness to Participate in a Discontinuation Trial. Drugs Aging 36, 1133–1139 (2019). https://doi.org/10.1007/s40266-019-00712-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00712-6