Abstract
Sarecycline (Seysara™) is an oral, once-daily, tetracycline-class drug for which a tablet formulation is approved in the USA for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients aged ≥ 9 years. The drug was developed by Paratek and Allergen and later acquired by Almirall S.A. (a Barcelona-based pharmaceutical company focused on medical dermatology). Sarceycline tablets were approved in early October 2018 and are planned to be available for patients in January 2019. Sarecycline capsules have also been studied in the USA, but no recent reports of development have been identified for this formulation. There are currently no clinical trials underway assessing sarecycline in rosacea. This article summarizes the milestones in the development of sarecycline leading to this first approval for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris.
Similar content being viewed by others
1 Introduction
Acne develops via a multifactorial process involving factors such as follicular hyperkeratinization, Propionibacterium acnes colonization, sebum production and inflammation [1]. For moderate to severe and inflammatory acne vulgaris, oral antibacterials are standard care components [1, 2], with tetracyclines and macrolides usually preferred [1,2,3]. However, these agents have certain limitations, among which are photosensitivity (tetracyclines), adverse vestibular effects (minocycline), gastrointestinal disturbances (particularly with macrolides and doxycycline) [1], dysbiosis [4] and microbial resistance concerns [5]. Additional oral antibacterials have therefore been investigated.

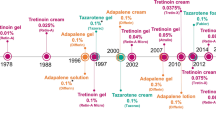
Key milestones in the development of sarecycline for the treatment of acne vulgaris, focussing on phase 3 trials. NDA new drug application
Sarecycline (Seysara™) is a new oral tetracycline-class antibiotic developed by Paratek and Allergan, and acquired by Almirall S.A., for the treatment of acne vulgaris. In October 2018 [6], the US FDA approved sarecycline tablets for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients aged ≥ 9 years [7]. Sarecycline tablets should be taken once daily (with or without food), with the recommended daily dose being based on the patient’s bodyweight (60 mg if 33–54 kg, 100 mg if 55–84 kg and 150 mg if 85–136 kg) [7]. Sarecycline capsules have also been studied in the USA, but no recent reports of development have been identified. There are currently no clinical trials underway assessing sarecycline in rosacea.
1.1 Company Agreements
In July 2007, Warner Chilcott (now Allergan, previously Actavis) entered an agreement to develop and commercialize certain narrow-spectrum tetracyclines originated by Paratek for the treatment of acne and rosacea [8]. Allergan (now Almirall) was responsible for their development and have exclusive rights to market them in the USA, while Paratek retains non-USA rights. Paratek received an up-front payment and will receive further payments at key milestones of development/regulatory approval as well as royalties on the product sales [8]. Almirall acquired most of the US dermatology portfolio of Allergan (which includes sarecycline) in August 2018; the deal was worth up to $US650 million, with $US550 million paid upfront and a potential earn-out in 2022 of up to $US100 million (depending on performance) [9]. The acquisition was finalized in September 2018 [10]. As of December 2016, the patent portfolio for Paratek's acne and rosacea programme (which encompasses compositions of matter, methods of use and sarecycline salts and polymorphs) included two issued US patents (8,318,706 and 8,513,223, which are expected to expire in 2031 and 2029) and corresponding foreign national or regional counterpart applications [11].
2 Scientific Summary
2.1 Pharmacodynamics
Sarecycline is a ribosomal protein inhibitor of the tetracycline class that displays potent activity against P. acnes and other Gram-positive bacteria in vitro [12]. The drug has also demonstrated anti-inflammatory effects in vitro [12]. These properties appear to be consistent with those of other tetracyclines, although the exact mechanism by which sarecycline acts to treat acne vulgaris is currently unknown [7]. The drug was not associated with clinically relevant QT interval prolongation when used at a dose approximately threefold greater than the recommended maximum [7].
Sarecycline (like other tetracyclines) may impact the bactericidal effects of penicillin; coadministration should therefore be avoided [7]. Coadministering sarecycline with oral retinoids should also be avoided, as both tetracyclines and oral retinoids can increase intracranial pressure. Plasma prothrombin activity may be reduced by sarecycline (as with other tetracyclines) which could elevate the bleeding risk of patients taking anticoagulants; the dosage of the anticoagulant may therefore need to be reduced [7].
Some recipients of teracyclines can experience photosensitivity [7] and sarecycline has displayed photoxic potential in mice [13]; patients should be advised to avoid/minimize exposure to sunlight (natural and artificial) while taking sarecycline [7]. In animal toxicity studies of oral sarecycline, pigment deposition in the thyroid gland or tooth/bone discolouration were not considered to be toxicologically adverse [13]; sarecycline should not be used during tooth development [7].
2.2 Pharmacokinetics
Sarecycline reaches maximum plasma concentrations (Cmax) in a median time of 1.5–2.0 h; the drug reaches steady state by day 7 and has a mean accumulation ratio of 1.5- to 1.6-fold with repeat dosing [7]. Steady-state exposure to the drug in healthy subjects increased slightly less than proportionally when the once-daily dose was increased from 60 to 150 mg. Sarecycline can be taken with or without food, although administration of the drug with a milk-containing meal high in fat and calories reduced exposure to the drug by ≈ 30% and delayed the time to Cmax by ≈ 0.53 h [7], consistent with some other tetracycline-class drugs. Sarecycline is 62.5–74.7% protein bound in vitro and has a mean apparent volume of distribution of 91.4–97.0 L at steady state. Sarecycline is minimally metabolized by liver microsome enzymes in vitro (< 15%); non-enzymatic epimerization, demethylation, hydroxylation and desaturation produce minor metabolites. The drug is excreted via both the faeces and the urine, with 42.6 and 44.1% of a single 100 mg oral dose being recovered via these routes (14.9 and 24.7% as unchanged sarecycline). Sarecycline has a mean elimination half-life of 21–22 h and its mean apparent oral clearance is ≈ 3 L/h at steady state [7].

Chemical structure of sarecycline
The pharmacokinetics of sarecycline are not impacted to any clinically relevant extent by age, sex, body weight, renal impairment or mild to moderate hepatic impairment (Child Pugh class A or B) [7]. The pharmacokinetics of sarecycline have not been assessed in the setting of severe hepatic impairment (Child Pugh class C) or end-stage renal disease [7].
As with other tetracyclines, sarecycline absorption may be impaired by iron-containing preparations, aluminium-, calcium- or magnesium-containing antacids or bismuth subsalicylate, necessitating separate administration [7]. Sarecycline inhibits p-glycoprotein in vitro and may therefore increase concentrations of drugs that are p-glycoprotein substrates; dosage reduction and toxicity monitoring may be required if these agents are used in combination. Sarecycline does not inhibit other key transporter proteins (BCRP, OATP1B1, OATP1B3, OAT1, OAT3 or OCT2) or key CYP isoenzymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4/5) and is not an inducer of CYP1A2, CYP2B6 or CYP3A4/5 in vitro [7].
2.3 Therapeutic Trials
Sarecycline has been studied in numerous phase 1 trials, one dose-ranging phase 2 trial, two 12-week phase 3 trials (in ≈ 2000 patients) and one 40-week phase 3 safety extension study, with the phase 2 and 3 studies being the focus here. No active comparator-controlled trials have been conducted with sarecycline.
2.3.1 Phase 3
After 12 weeks of treatment in a phase 3 trial in patients aged 9–45 years with moderate to severe facial acne vulgaris (study SC1401; NCT02320149), significantly more recipients of once-daily sarecycline 1.5 mg/kg (as tablets) [n = 483] than of placebo (n = 485) achieved treatment success on the face (21.9 vs. 10.5%; p < 0.0001), defined as a ≥ 2-point decrease (improvement) from baseline in Investigator’s Global Assessment (IGA) score plus an IGA score of 0 or 1 (i.e. clear or almost clear) [14] [primary endpoint [7]]. The facial success rate in this randomized, double-blind trial began to significantly favour sarecycline over placebo after 9 weeks of treatment [14]. These findings were supported by changes from baseline in lesion counts at week 12, including the mean absolute change in inflammatory lesions (− 15.3 with sarecycline vs. − 10.2 with placebo; between-group statistical analyses not reported) [primary endpoint] [7], the least-squares mean (LSM) percentage change in inflammatory lesions (− 51.8 vs. − 35.1%; p < 0.0001) [14] and the LSM absolute change in non-inflammatory lesions (− 15.1 vs. − 11.2%; p < 0.01) [14]. The benefit of sarecycline over placebo was evident as early as week 3 of treatment for the inflammatory lesion count [7, 14] and week 6 of treatment for the non-inflammatory lesion count [14].
For those affected, acne on the chest and back also improved with sarecycline, with the rates of success (i.e. ≥ 2-point decrease in IGA score) at 12 weeks being greater (p < 0.05) with the drug than with placebo (29.6 vs. 19.6% and 32.9 vs. 17.1%) [post hoc] [14]. Sarecycline was also associated with health-related quality of life benefits, being significantly more favourable than placebo for the symptoms, emotion and total (but not functioning) score of the Skindex-16 questionnaire. At baseline, the sarecycline and placebo groups had a mean of 29.7 and 30.2 inflammatory facial lesions and a mean of 42.4 and 43.7 non-inflammatory facial lesions; overall, across groups, 39 and 55% of patients had chest or back acne (IGA score ≥ 2 at these locations) [14].
These findings were supported by an identically-designed phase 3 study (SC1402; NCT02322866) [14]. The rate of facial treatment success was significantly (p = 0.0038) greater with sarecycline (n = 519) than with placebo (n = 515) after 12 weeks of treatment (22.6 vs. 15.3%) [14] (primary endpoint [7]), with the difference between the groups significantly favouring sarecycline after only 6 weeks of therapy [14]. Changes from baseline in lesion counts with sarecycline and placebo at week 12 were consistent with these findings, including the mean absolute change in inflammatory lesion count (− 15.5 vs. − 11.1; no between-group statistical analyses reported) [primary endpoint] [7], the LSM percentage change in inflammatory lesion count (− 49.9 vs. − 35.4%; p < 0.0001) [14] and the LSM absolute change in non-inflammatory lesion count (− 16.2 vs. − 13.4%; p < 0.01) [14]. Sarecycline was more favourable than placebo from week 3 of treatment onwards for each inflammatory lesion measure [7, 14] and week 9 onwards for the non-inflammatory lesion measure [14]. Moreover, for the 48 and 62% of patients with chest or back acne at baseline (IGA score ≥ 2), the rate of success at these sites at 12 weeks was greater (p < 0.05) with sarecycline than with placebo (36.6 vs. 21.6% and 33.2 vs. 25.7%) [post hoc] [14]. Health-related quality of life measures at 12 weeks were also significantly more favourable with sarecycline than with placebo, including the symptoms, emotional, functioning and total scores of the Skindex-16 questionnaire. At baseline, the sarecycline and placebo groups had a mean facial inflammatory lesion count of 30.3 and 30.2, respectively, and a mean facial non-inflammatory lesion count of 42.3 and 43.9 [14].
Patients who participated in SC1401 and SC1402 could receive sarecycline 1.5 mg/kg once daily in a longer-term phase 3 study (NCT02413346; SC1403) [15]. This study (n = 483) was open-label and designed to assess safety [15]; no efficacy data are available at present.
2.3.2 Phase 2
Various once-daily doses of sarecycline (0.75, 1.5 and 3.0 mg/kg) were assessed in the treatment of moderate to severe facial acne vulgaris in patients aged 12–45 years in a phase 2 trial (PR-10411; NCT01628549) [16]. Sarecycline 1.5 and 3.0 mg/kg once daily reduced inflammatory lesion counts, being significantly (p < 0.05) more favourable than placebo for both the percentage LSM change from baseline (− 52.7 and − 51.8 vs. − 38.3%) and the absolute LSM change from baseline (− 16.9 and − 16.8 vs. − 12.5) after 12 weeks of treatment; corresponding changes with sarecycline 0.75 mg/kg once daily (− 42.5% and − 14) were not significant versus placebo. None of the sarecycline dosages (0.75, 1.5 or 3.0 mg/kg once daily) displayed significant benefit over placebo in terms of non-inflammatory lesion count changes at this timepoint (respective groups had LSM percentage changes from baseline of − 34.9, − 37.5 and − 32.3 vs. − 35.2% and LSM absolute changes from baseline of − 18, − 19.4 and − 17.6 vs. − 17.9). In this randomized, double-blind trial, a total of 284 patients were evaluable for efficacy, including 76, 70 and 66 in the sarecycline 0.75, 1.5 and 3.0 mg/kg groups and 72 in the placebo group. Across the groups, the mean inflammatory lesion count was 32.0–33.5 and the mean non-inflammatory lesion count was 50.6–54.6. Sarecycline was administered as capsules [16].
2.4 Adverse Events
Sarecycline tablets administered at a dosage of 1.5 mg/kg once daily for 12 weeks were generally well tolerated in patients aged 9–45 years with moderate to severe facial acne vulgaris in the SC1401 and SC1402 phase 3 trials [14]. The incidence of treatment-related adverse events (TRAEs) in the sarecycline and placebo group was 10.6 and 8.7% in SC1401 and 8.0 and 5.7% in SC1402. Treatment-emergent adverse events (TEAEs) were generally mild or moderate and the most common that occurred with a numerically greater incidence with sarecycline than with placebo was nausea (4.6 vs. 2.5% in SC1401; 1.9 vs. 1.0% in SC1402). Few TEAEs were serious (≤ 1.0% of patients in any group), none of which were considered to be related or possibly related to treatment, and no patients died in either trial. Study discontinuation because of TEAEs was also uncommon (≤ 2.1% of patients in any group), with most of these TEAEs being considered possibly related (SC1401 and SC1402) or related (SC1402) to study therapy. Other tetracycline-class antibiotics are often associated with certain gastrointestinal, vestibular and phototoxic adverse effects and, in females, vaginal yeast infections, although these TEAEs were generally uncommon with sarecycline. Among those that occurred with a numerically greater incidence with sarecycline than with placebo in SC1401 and/or SC1402 (nausea, vomiting, abdominal pain, abdominal discomfort, sunburn, vulvovaginal candidiasis and vulvovaginal mycotic infection), most occurred in < 1.5% of sarecycline recipients, with the exception of nausea (in SC1401 and SC1402; see earlier discussion) and vomiting (in SC1401; 2.1 vs. 1.4% of placebo recipients) [14].
The short-term tolerability of sarecycline is further supported by the phase 2 PR-10411 study that assessed sarecycline capsules at once-daily doses of 0.75, 1.5 or 3.0 mg/kg over 12 weeks [16]. For instance, TRAEs occurred in 13.2% of sarecycline recipients (all doses pooled) and 9.6% of placebo recipients, the most common of which was nausea, although its incidence was numerically lower with sarecycline than with placebo (3.3 vs. 4.1%). TEAEs rarely led to discontinuation of therapy (one sarecycline 0.75 mg/kg recipient, four sarecycline 3.0 mg/kg recipients and one placebo recipient), with those considered to be TRAEs with sarecycline including hypoaesthesia, decreased white blood cell count and increased blood creatine phosphokinase [16].
Longer term, most participants of SC1401 and SC1402 who entered the SC1403 safety trial experienced TEAEs with sarecycline 1.5 mg/kg once daily over a period of up to 40 weeks, regardless of whether they had originally received sarecycline (n = 247) or placebo (n = 236) in the parent trial (94% in each group) [primary outcome measure] [15]. Patients in SC1403 received sarecycline until their facial acne had adequately improved and could re-start the drug if the acne returned [15].
2.5 Ongoing Clinical Trials
We are not aware of any sarecycline trials that are currently ongoing.
3 Current Status
Sarecycline received its first global approval in October 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients aged ≥ 9 years in the USA [6].
Change history
29 April 2019
The article Sarecycline: First Global Approval, written by Emma D. Deeks, was originally published Online First without open access. After publication in volume 79, issue 3, pages 325–329 Almirall, LLC., requested that the article be Open Choice to make the article an open access publication. Post-publication open access was funded by Almirall, LLC.
References
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973 e33.
Hauk L. Acne vulgaris: treatment guidelines from the AAD. Am Fam Physician. 2017;95(11):740–1.
Mayo Clinic. Acne. 2017. https://www.mayoclinic.org/. Accessed 18 Oct 2018.
Del Rosso JQ, Gallo RL, Thiboutot D, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society. Part 2: perspectives on antibiotic use and the microbiome and review of microbiologic effects of selected specific therapeutic agents commonly used by dermatologists. J Clin Aesthet Dermatol. 2016;9(5):11–7.
Sinnott SJ, Bhate K, Margolis DJ, et al. Antibiotics and acne: an emerging iceberg of antibiotic resistance? Br J Dermatol. 2016;175(6):1127–8.
U.S. Food and Drug Administration. Novel drug approvals for 2018. 2018. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm592464.htm. Accessed 18 Oct 2018.
Allergan Pharmaceuticals International Limited. Seysara (Sarecycline) tablets for oral use: US prescribing information. 2018. https://www.accessdata.fda.gov/. Accessed 18 Oct 2018.
Warner Chilcott. Warner Chilcott and Paratek Pharmaceuticals sign collaboration agreement for novel, narrow-spectrum agents for acne and rosacea [media release]. 9 July 2007. http://www.warnerchilcot.com.
Almirall Canada. Almirall buys Allergan’s US dermatology portfolio for $650 million [media release]. 3 Aug 2018. http://www.almirall.com.
Almirall S.A. Almirall acquires Allergan U.S. medical dermatology portfolio [media release]. 21 Sep 2018. http://www.almirall.com.
U.S. Securities and Exchange Commission. Form 10-K: Paratek Pharmaceuticals, Inc. 2016. https://www.sec.gov/Archives/edgar/data/1178711/000156459017003144/prtk-10k_20161231.htm. Accessed 18 Oct 2018.
Data on file, Allergan plc, 2018.
Center for Drug Evaluation and Research. Application number 209521Orig1s000. Multi-discipline review. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/209521Orig1s000MultidisciplineR.pdf. Accessed 10 Jan 2019.
Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17(9):987–96.
US National Institutes of Health. ClinicalTrials.gov identifier NCT02413346. 2018. https://clinicaltrials.gov. Accessed 17 Oct 2018.
Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17(3):333–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Emma Deeks is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Deeks, E.D. Sarecycline: First Global Approval. Drugs 79, 325–329 (2019). https://doi.org/10.1007/s40265-019-1053-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-1053-4