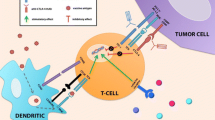
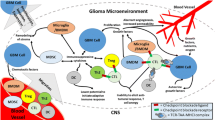
Abstract
Immunotherapeutic approaches have been, and continue to be, aggressively investigated in the treatment of infiltrating gliomas. While the results of late-phase clinical studies have been disappointing in this disease space thus far, the success of immunotherapies in other malignancies as well as the incremental gains in our understanding of immune-tumour interactions in gliomas has fuelled a strong continued interest of their evaluation in these tumours. We discuss a range of immunotherapeutic approaches including, but not limited to, vaccines, checkpoint inhibitors, oncolytic viruses, and gene therapies. Potential biomarkers under investigation to help elucidate which patients may respond or not respond to immunotherapeutic regimens are reviewed. Directions for future investigations are also noted.
Similar content being viewed by others
References
Lukas RV, Mrugala MM. Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neuro Oncol Pract. 2017;4(4):209–19.
Lukas RV, Wainwright DA, Ladomersky E, Sachdev S, Sonabend AM, Stupp R. Newly diagnosed glioblastoma: a review on clinical management. Oncology (Williston Park). 2019;33(3):623491.
Mrugala MM, Ruzevick J, Zlomanczuk P, Lukas RV. Tumor treating fields in neuro-oncological practice. Curr Oncol Rep. 2017;19(8):53.
Kim WJ, Dho YS, Ock CY, et al. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol. 2019;143(2):321–8.
Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–68.
Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000–7.
Lin AJ, Campian JL, Hui C, et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol. 2018;136(2):403–11.
Campian JL, Piotrowski AF, Ye X, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. 2017;135(2):343–51.
Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Cancer Netw. 2015;13(10):1225–31.
Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127(2):329–35.
Hegi ME, Stupp R. Witholding temozlomide in glioblastoma patients with unmethylated MGMT promoter—still a dilemma? Neuro Oncol. 2015;17(11):1425–7.
Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–33.
Weller M, Butowski N, Tran DD, et al. Rindopepimut and temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–85.
Cloughesy TF, Mochizuki AY, Oprilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86.
Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–6.
Choi S, Yu Y, Grimmer MR, Wahl M, Chang SM, Costello JF. Temozlomide-associated hypermutation in gliomas. Neuro Oncol. 2018;20(10):1300–9.
Van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129(4):597–607.
Di Tomaso T, Mazzoleni S, Wang E, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16(3):800–13.
Wu A, Wiesner S, Xiao J, et al. Expression of MHC I and NK ligands on human CD133 + glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83(2):133.
Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Part 5):1458–71.
Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51.
Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin 12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505):eaaw5680.
Saito T, Sugiyama K, Hama S, et al. Prognostic importance of temozolomide-induced neutropenia in glioblastoma. IDH-wildtype patients. Neurosurg Rev. 2018;41(2):621–8.
Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114(1):149–54.
Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–8.
Back M, Jayamane D, Brazier D, et al. Tumor volume reduction following PET guided intensity modulated radiation therapy and temozolomide in IDH mutated anaplastic glioma. J Clin Neurosci. 2019;59:68–74.
Ladomersky E, Zhai L, Lenzen A, et al. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res. 2018;24(11):2559–73.
Zhang D, Qiu B, Wang Y, Guan Y, Zhang L, Wu A. Temozolomide increases MHC-I expression via NF-κB signaling in glioma stem cells. Cell Biol Int. 2017;41(6):680–90.
Galluzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714.
Kirson ED, Giladi M, Gurvich Z, et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis. 2009;26(7):633–40.
Voloshin T, Yitzhaki OT, Kaynan N, et al. Tumor treating fields (TTFields) plus anti-PD-1 therapy induce immunogenic cell death resulting in enhanced antitumor efficacy. AACR abstract 3665. Cancer Res. 2017;77(13):3665.
Ladomersky E, Genet M, Zhai L, et al. Improving vaccine efficacy against malignant glioma. Oncoimmulogy. 2016;5(8):e1196311.
Weller M, Roth P, Preusser M, et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13(6):363–74.
Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II single-arm trial. Neuro Oncol. 2014;16(2):274–9.
Bloch O, Shi Q, Anderson SK, et al. ATIM-14. Alliance A071101: a phase 2 randomized trial comparing the efficacy of heat shock protein peptide complex-96 (HSPPC-96)vaccine given with bevacizumab versus bevacizumab alone in the treatment of surgically resectable recurrent glioblastoma. Neuro Oncol. 2017;19(suppl_6):vi29.
Liau LM, Ashkan A, Tran KDD, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142.
Liau LM, Ashkan K, Tran DD, et al. Correction to: First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):179.
Johanns TM, Bowman-Kirigin JA, Liu C, Dunn GP. Targeting neoantiegns in glioblastoma: an overview of cancer immunogenomics and translational implications. Neurosurgery. 2017;64(CN_suppl_1):165–76.
Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase 1b glioblastoma trial. Nature. 2019;565(7738):234–9.
Reardon DA, Schuster J, Tran DD, et al. ReACT: Overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. J Clin Oncol. 2015;33(15_suppl):2009.
Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–5.
Platten M, Schilling D, Bunse L, et al. NOA-16: A first-in-man multicenter phase 1 clinical trial of the German Neurooncology Working Group evaluating a mutation-specific peptide vaccine targeting IDHR132H in patients with newly diagnosed malignant astrocytomas ATIM-33. Neuro Oncol. 2018;20(suppl_6):vi8–9.
Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumor immunity. Nature. 2014;512(7514):324–7.
Reardon DA OA, Brandes AA, Rieger J, Wick A, Sepulveda J, Phuphanich S, De Sousa P, Ahluwalia MS, Lim M, Vlahovic G, Sampson J. Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-Oncology. 2017;19(Suppl. 3):iii21.
Bristol-Meyers Squibb press release. 2019. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did. Accessed 9 May 2019.
Bristol-Meyers Squibb press release. 2019. https://investors.bms.com/iframes/press-releases/press-release-details/2019/Bristol-Myers-Squibb-Provides-Update-on-Phase-3-Opdivo-nivolumab-CheckMate–548-Trial-in-Patients-with-Newly-Diagnosed-MGMT-Methylated-Glioblastoma-Multiforme/default.aspx. Accessed 5 Sept 2019.
Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–42.
Lukas RV, Rodon J, Becker K, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. 2018;140(2):317–28.
Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–6.
Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–9.
Panek WK, Kane JR, Young JS, et al. Hitting the nail on the head: combining adenovirus-mediated virotherapy and immunomodulation for the treatment of glioma. Oncotarget. 2017;8(51):89391–405.
Desjardins A, Gromeier M, Herndon JE 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–61.
Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (delta-24-RGD) oncoloytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–27.
Kloeppinger L, Amidei C, Lesniak M, et al. QOLP-11. Quality of life in high-grade glioma patients on a phase 1 virotheapy study. Neuro Oncol. 2018;20(suppl_6):vi216.
Migliorini D, Dietrich PY, Stupp R, Linette GP, Posey AD Jr, June CH. CAR-T cell therapies in glioblastoma. Clin Cancer Res. 2018;24(3):535–40.
Chuntova P, Downey KM, Hegde B, Almeida ND, Okada H. Genetically engineered T-cells for malignant glioma: overcoming the barriers to effective immunotherapy. Front Immunol. 2019;9:3062.
O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984.
Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–18.
Joseph SK, Samaha H, Bielamowicz K, Ahmed N, et al. Response to the comment on “Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma” by Bielamowicz. Neuro Oncol. 2018;20(7):1004–5.
Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–9.
Goff SL, Morgan RA, Yang JC, et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother. 2019;42(4):126–35.
Van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17(7):935–41.
Gresser I. Interferon and cancer: therapeutic prospects. Rev Eur Etud Clin Biol. 1970;15(1):23–7.
Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301.
Margolin KA, Rayner AA, Hawkins MJ, et al. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989;7(4):486–98.
Dixit K, Kumthekar P. Gene delivery in neuro-oncology. Curr Oncol Rep. 2017;9(11):69.
Chiocca EA, Smith KM, McKinney B, et al. A phase 1 trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16(3):618–26.
Chiocca EA, Lukas R, Rao G, Barrett JA, Buck JY, Demars N, Smith A, Miao J, Zhou J, Gelb A, Cooper LJN. Evaluation of controlled IL-12 in combination with a PD-1 inhibitor in subjects with recurrent glioblastoma. J Clin Oncol. 2019;37(15 suppl):2020.
Cloughesy TF, Landolfi J, Vogelbaum MA, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20(10):1383–92.
Tocagen reports results of Toca 5 phase 3 trial in recurrent brain cancer. 2019. https://ir.tocagen.com/news-releases/news-release-details/tocagen-reports-results-toca-5-phase-3-trial-recurrent-brain. Accessed 12 Sept 2019.
Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205.
Pratt D, Dominah G, Lobel G, et al. Programmed death ligand 1 is a negative prognostic marker in recurrent isocitrate dehydrogenase-wildtype glioblastoma. Neurosurgery. 2019;85(2):280–89.
Gorsi HS, Malicki DM, Barsan V, et al. Nivolumab in the treatment of recurrent or refractory pediatric brain tumors: a single institutional experience. J Pediatr Hematol Oncol. 2019;41(4):e235–41.
Larouche V, Atkinson J, Albrecht S, et al. Sustained complete response of recurrent glioblastoma to combined checkpoint inhibition in a young patient with constitutional mismatch repair deficiency. Pediatr Blood Cancer. 2018;65(12):e27389.
Zhao J, Chen AX, Gartell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–9.
Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8.
Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Vredenurgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15(12):1329–34.
Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–71.
Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13(11):1771–5.
Ladomersky E, Scholtens DM, Kocherginsky M, et al. The coincidence between increasing age, immunosuppression, and the incidence of patients with glioblastoma. Front Pharmacol. 2019;10:200.
Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflamm. 2019;16(1):53.
Otto-Meyer S, Lumibao J, Kim E, et al. The interplay among psychological distress, the immune system, and brain tumor patient outcomes. Curr Opin Behav Sci. 2019;28:44–50.
Kokolus KM, Capitano ML, Lee CT, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110(50):20176–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
RVL, DAW, CMH, AMS are supported by P50CA221747 SPORE for Translational Approaches to Brain Tumours. DAW and RVL are supported by BrainUp grant 2136.
Conflicts of interest
RVL has received honoraria for serving on advisory boards for Monteris and Ziopharm. RVL, CMH, AMS, and FMI have received honoraria as consultants for Abbvie. FMI has received honoraria for consulting for Merck, Novocure, Tocagen, Alexion, and Regeneron. RVL has received support from Roche for travel to present at a meeting. FMI has received travel support to FDA meeting from Merck. FMI has received funding for research support for investigator-initiated trials from Merck, BMS, and Novocure. RVL have received honoraria for medical editing from Medlink Neurolology, medical review of content for EBSCO Publishing, and creating/presenting board review material for American Physician Institute.
Rights and permissions
About this article
Cite this article
Lukas, R.V., Wainwright, D.A., Horbinski, C.M. et al. Immunotherapy Against Gliomas: is the Breakthrough Near?. Drugs 79, 1839–1848 (2019). https://doi.org/10.1007/s40265-019-01203-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01203-z