Abstract
Sodium zirconium cyclosilicate (Lokelma™) [hereafter referred to as SZC] is a non-absorbed, non-polymer zirconium silicate compound that preferentially exchanges hydrogen and sodium for potassium and ammonium ions in the gastrointestinal tract (GIT), thereby increasing faecal potassium excretion and lowering serum potassium levels. It is available as a powder for oral suspension (in water) and is approved in the EU and the USA for the treatment of hyperkalaemia in adults. In two multinational, phase III studies in adults with hyperkalaemia, SZC 10 g three times daily lowered serum potassium levels to within the normal range (3.5–5.0 mmol/L) during the first 48 h of treatment, and SZC 5 and 10 g once daily maintained normokalaemia over ≤ 28 days’ therapy. These beneficial effects were consistent across all patient subgroups (e.g. chronic kidney disease, diabetes, heart failure, concomitant use of RAAS inhibitor therapy), and appeared to be maintained over the longer term (≤ 12 months). SZC was generally well tolerated in adults with hyperkalaemia. Its tolerability profile was generally similar to that seen with placebo over ≤ 28 day, and its safety profile appeared to remain consistent over the longer term (≤ 12 months). Moreover, the incidence of hypokalemia was low. Current evidence indicates that SZC is a promising therapy for the management of hyperkalaemia in adults.
Similar content being viewed by others
Non-polymer zirconium silicate compound that entraps potassium and ammonium ions and exchanges them for hydrogen and sodium ions in the GIT |
Lowers serum potassium levels to within the normal range during the first 48 h of treatment and maintains normokalaemia over the longer term (≤ 12 months) |
Effective regardless of chronic kidney disease, diabetes, heart failure and concomitant use of RAAS inhibitors |
Low incidence of hypokalemia |
1 Introduction
Potassium levels within the body are balanced by two concurrent processes: external homeostasis regulates renal potassium excretion to balance intake (dietary and supplemental), extra-renal loss and deficits, while internal homeostasis involves the distribution of total body potassium [1]. Potassium is asymmetrically distributed under physiological conditions; approximately 98% is located in the intracellular space, with only 2% located in the extracellular space [1, 2]. This gradient is crucial for the maintenance of various physiological processes, including resting membrane potential, and thus, extracellular (serum) potassium levels are strictly regulated within narrow limits (3.5–5.0 mmol/L) [2].
The cellular uptake (e.g. in the liver and muscle) and renal excretion (predominantly by the aldosterone-sensitive distal nephron) of potassium act to buffer against acute increases in potassium levels above the normal range (i.e. acute hyperkalaemia) [3]. Chronic hyperkalaemia usually denotes a defect in the handling of renal potassium [3]. Hyperkalaemia is common in patients with renal impairment [4], with several factors, including a serum potassium level of ≥ 4.5 mmol/L, an eGFR of < 45 mL/min/1.73 m2, diabetes and heart failure, increasing the risk of hyperkalaemia in patients with chronic kidney disease (CKD) [5]. This risk is further increased with the use of RAAS inhibitors, which are used to slow nephropathy progression to end-stage renal disease, but which are often discontinued or administered at suboptimal doses in many patients due to concerns over the development of hyperkalaemia [4, 5].
The management of hyperkalaemia may involve, among other actions, redistributing extracellular potassium to the intracellular space (with a β2-adrenergic receptor agonist, or glucose and insulin) or promoting potassium excretion via the blood (with haemodialysis), urine (with diuretics) or gastrointestinal tract (GIT) [with potassium-binding agents] [2, 6, 7]. Potassium-binding agents act by increasing faecal potassium excretion [2, 7]. One such recently developed agent is the non-absorbed, cation exchanger sodium zirconium cyclosilicate (Lokelma™) [hereafter referred to as SZC], which is available as a powder for oral suspension (in water) [8, 9]. SZC is approved in the EU [8] and the USA [9] for the treatment of hyperkalaemia in adults. This article discusses pharmacological, therapeutic efficacy and tolerability data relevant to the use of SZC for the treatment of hyperkalaemia in adults, with a focus on the approved EU dosages (see Sect. 5), which differ from those approved in the USA.
2 Pharmacological Properties of SZC
SZC is an insoluble, inorganic, non-polymer zirconium silicate compound comprising units of oxygen-linked zirconium and silicon atoms in the form of a microporous cubic lattice framework [8,9,10]. The bonds between the oxygen and zirconium or silicon atoms are mostly covalent, with the octahedral [ZrO6]−2 units conferring the negative charge that enables cation exchange [10]. The size of the pore openings is estimated to be approximately 3Å (i.e. approximately the diameter of an unhydrated potassium ion) [10] (Fig. 1).

Adapted from Tamargo et al. [2] with permission
Pore detail structure of sodium zirconium cyclosilicate with an entrapped potassium ion (K+). Blue spheres indicate oxygen atoms, green spheres indicate silicon atoms and red spheres indicate zirconium atoms.
SZC selectively entraps monovalent cations (specifically potassium and ammonium) over divalent cations (such as calcium and magnesium) and exchanges them for hydrogen and sodium in the GIT [8,9,10]. In vitro, SZC was > 25-fold more selective for potassium ions over calcium and magnesium ions, with the exchange capacity of SZC for calcium and magnesium ions being below 0.05 mEq/g [i.e. the lower limit of quantification (LLOQ)] [10]. In contrast, the organic polymer resin sodium polystyrene sulfonate (SPS) only showed 0.2–0.3-fold greater selectivity for potassium ions over calcium and magnesium ions. In media buffered to mimic the pH of the human GIT, SZC was associated with a rapid (within 5 min) uptake of potassium ions, mostly in simulated small intestinal and large intestinal fluids, with potassium equilibrium reached in < 20 min [10].
By binding potassium ions, SZC lowers free potassium levels in the lumen of the GIT, thereby increasing faecal potassium excretion and lowering serum potassium levels [8, 9]. SZC 5 and 10 g once daily for 4 days increased faecal potassium excretion and decreased serum potassium levels and (as a consequence of the reduction in serum potassium levels) urinary potassium excretion in a dose-dependent manner, according to a phase 1 study (ZS-006) in healthy adult volunteers receiving a standardized low sodium and high potassium diet [8, 9]. In patients with hyperkalaemia participating in a phase III study (ZS-003; discussed in Sect. 3.1), SZC demonstrated dose-dependent reductions in serum potassium levels across the 2.5–10 g dose range [9].
SZC has not been shown to affect serum calcium or magnesium levels, and has demonstrated no consistent effect on systolic and diastolic blood pressure [8] (see Sect. 4). It has been shown to bind ammonium ions in vitro and in vivo, thereby removing ammonium and increasing serum bicarbonate levels [8] (see Sect. 4). SZC was associated with significant (p < 0.05) reductions in serum aldosterone levels, according to a prespecified analysis (n = 181) of data (available as an abstract) [11] from a phase III study (HARMONIZE; discussed in Sect. 3.1).
No studies have been conducted to compare the pharmacodynamic properties of SZC when administered with or without food [8]. SZC does not absorb water (i.e. swell), which may account for its low GIT adverse event profile [12] (see Sect. 4).
SZC was not systemically absorbed in clinical studies [8], with zirconium concentrations in the blood and urine being similar between treated and untreated patients with hyperkalaemia (i.e. undetectable or around the LLOQ) [9]. Thus, the risk of systemic toxicity is very low [10]. SZC is not subject to enzymatic metabolism [8, 9]. As SZC is not absorbed or metabolized, its effects on the function of, or binding to, other agents is limited [8]. However, it can transiently increase gastric pH (by absorbing hydrogen ions) and, therefore, may change the absorption and thus potentially the efficacy or safety of concomitant agents that exhibit pH-dependent solubility [8, 9].
3 Therapeutic Efficacy of SZC
A first-in-human, randomized, double-blind, multicentre, phase II, dose-escalation study [13] in 90 adults (aged ≥ 18 years) with serum potassium levels of 5.0–6.0 mmol/L and stable stage 3 CKD [estimated glomerular filtration rate (eGFR) of 30–60 mL/min/1.73 m2] evaluated the therapeutic efficacy of SZC [0.3, 3 or 10 g (n = 12, 24 and 24), administered as an oral suspension (in 180 mL of water) three times daily with food] compared with placebo (n = 30) over 48–96 h. Compared with placebo, the approved 10 g three times daily starting dosage of SZC met the primary endpoint (the rate of change from baseline in serum potassium levels in the first 48 h) in the overall population and the subgroup of patients receiving renin–angiotensin–aldosterone system (RAAS) inhibitors (each p < 0.0001) [8, 13]. Moreover, after 38 h of treatment, the maximum mean reduction from baseline in serum potassium levels was 0.92 mmol/L (p < 0.001 vs. placebo); the magnitude of this reduction was considered clinically relevant, as a reduction of ≥ 1 mmol/L at 48 h in post-critical care may reduce the mortality associated with hyperkalaemia and mortality [13].
Two randomized, double-blind, placebo-controlled, multinational, phase III studies (ZS-003 [14] and ZS-004 (HARMONIZE) [15]) evaluated the therapeutic efficacy of SZC in adult outpatients with serum potassium levels of 5.0–6.5 mmol/L [14] or ≥ 5.1 mmol/L [15] (Sect. 3.1). Patients undergoing dialysis, those with diabetic ketoacidosis or those with cardiac arrhythmias requiring immediate treatment were among those excluded from these studies [14, 15]. Preliminary longer-term efficacy data from an 11-month extension of HARMONIZE [ZS-004E (NCT02107092)] and a ≤ 12-month, open-label, noncomparative, multinational, phase III study (ZS-005) are also reviewed (Sect. 3.2).
3.1 ZS-003 and HARMONIZE
ZS-003 and HARMONIZE each consisted of a 48-h (randomized [14] or open-label [15]) period followed by a 12-day [14] or 28-day [15] (randomized) maintenance period. In ZS-003, patients received SZC [1.25, 2.5, 5 or 10 g, administered as an oral suspension (in ≈ 180 mL of water) three times daily with food] or placebo for 48 h [14]. Those in the SZC group achieving normokalaemia (i.e. a serum potassium level of 3.5–5.0 mmol/L) then received either their original SZC dose (administered once daily before breakfast) or placebo, while those in the placebo group received SZC (1.25 or 2.5 g, administered once daily before breakfast) for 12 days [9, 14]. In HARMONIZE, patients received SZC [10 g, administered as an oral suspension (in 240 mL of water) three times daily with food] for 48 h [15]. Those achieving normokalaemia then received SZC (5, 10 or 15 g once daily) or placebo for 28 days. Patients with a serum potassium level of 3.0–3.4 mmol/L during the maintenance period had their dose reduced to once every other day for the remainder of the study. A serum potassium level of < 3.0 mmol/L at any time during the study, or a serum potassium level of > 6.2 mmol/L or significant cardiac arrhythmias during the maintenance period resulted in study discontinuation [15].
Apart from the serum potassium level, baseline characteristics did not significantly differ between the treatment groups in ZS-003 [14]. Overall, 75% of 753 patients had an eGFR of < 60 mL/min/1.73 m2, 67% were receiving RAAS inhibitors, 60% had diabetes and 40% had a history of heart failure, and the mean baseline serum potassium level was 5.3 mmol/L [9, 14]. In HARMONIZE, 70, 66, 66 and 36% of 258 patients were receiving RAAS inhibitors or had CKD, diabetes or heart failure, respectively, and the mean baseline serum potassium level was 5.6 mmol/L [15]. Of note, heart failure was broadly defined using a list of custom terms that differs somewhat from the list used to define heart failure in the international Medical Dictionary for Regulatory Activities [16]. Analyses were conducted in the intent-to-treat population [14, 15].
3.1.1 Correction Period
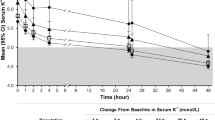
SZC 10 g three times daily was effective in lowering serum potassium levels to within the normal range during the first 48 h of treatment [14, 15]. It demonstrated a significant difference over placebo in the mean exponential rate of change from baseline in the serum potassium level per hour (primary endpoint) in ZS-003 (Table 1) [14]. The mean exponential rate of change from baseline in the serum potassium level per hour (assessed in 258 patients) in HARMONIZE (− 0.3%) [15] was consistent with that seen in ZS-003 (Table 1) [14]. The SZC 10 g three times daily dosage was also associated with a mean reduction from baseline in serum potassium levels at 48 h that was significant (p < 0.001) versus placebo in ZS-003 (Table 1) [14] and baseline in HARMONIZE (− 1.1 mmol/L) [15]. Significant (p ≤ 0.01) between-group differences favouring SZC 10 g three times daily over placebo [12, 14] and baseline [15] in this endpoint were seen as early as 1 h after the first dose and were apparent at all subsequent timepoints during the 48-h period.
According to prespecified subgroup analyses, SZC 10 g three times daily lowered serum potassium levels to within the normal range regardless of baseline serum potassium levels, eGFR, a history of CKD, diabetes or heart failure, and the concomitant use of RAAS inhibitor therapy [14, 15]. For instance, in a HARMONIZE subgroup of 94 patients with hyperkalaemia (mean serum potassium level at baseline of 5.6 mmol/L) and heart failure, therapy with SZC 10 g three times daily for 48 h resulted in a mean serum potassium level of 4.4 mmol/L (p < 0.001 vs. placebo) [17]. A significant (p < 0.001 vs. placebo) difference in this endpoint was seen as early as 1 h post dose [17]. Moreover, results (from an abstract) from a post hoc subgroup analysis of pooled data (n = 19) from ZS-003 and HARMONIZE found that SZC 10 g three times daily for 48 h significantly (p < 0.005) lowered serum potassium levels from baseline (mean serum value of 5.7 mmol/L) by 1.1 mmol/L in patients with hyperkalaemia who were receiving mineralocorticoid receptor antagonists [18]. A significant (p < 0.005) reduction from baseline in this endpoint was seen as early as 1 h after the first dose, with significant (p < 0.005) reductions apparent at all subsequent timepoints during the 48-h period [18]. Of note, as expected, patients with higher baseline serum potassium levels demonstrated a greater response to SZC therapy than those with lower baseline serum potassium levels [8, 9]. For instance, in ZS-003, SZC 10 g three times daily was associated with a mean reduction from baseline in serum potassium levels at 48 h of 1.1, 1.0 and 0.6 mmol/L in patients with a baseline serum potassium level of > 5.5, 5.4–5.5 and ≤ 5.3 mmol/L, respectively (n = 31, 36 and 90) [14]. Moreover, in HARMONIZE, reductions in serum potassium levels of 1.5, 1.2 and 0.8 mmol/L were seen in patients with baseline serum potassium levels of ≥ 6.0, 5.5–5.9 and < 5.5 mmol/L, respectively (n = 39, 100 and 119) [8, 15].
Most SZC 10 g three times daily recipients in ZS-003 (86.4%) [14] and HARMONIZE (98%) [15] achieved normokalaemia within 48 h, with a significantly greater proportion of SZC 10 g three times daily than placebo recipients achieving serum potassium levels within the normal range in ZS-003 (Table 1) [14]. Of note, the median time to normokalaemia in HARMONIZE was 2.2 h [15].
3.1.2 Maintenance Period
Serum potassium levels were maintained within the normal range with SZC 5 and 10 g once daily therapy, but increased to hyperkalaemic levels with placebo, over 12 [14] or 28 [15] days. SZC 5 and 10 g once daily each demonstrated a significant difference relative to placebo in the mean exponential rate of change from baseline in the serum potassium level per hour over the maintenance period (primary endpoint) in ZS-003 [14] and maintained significantly lower mean serum potassium levels during days 8–29 of the maintenance period (primary endpoint) than placebo in HARMONIZE [15] (Table 1). In HARMONIZE, SZC 5 and 10 g once daily maintained a significantly greater mean reduction from baseline of the correction period in serum potassium levels at day 29 than placebo (see Table 1) [15].
The proportions of patients with normokalaemia at day 29 or with mean serum potassium levels of < 5.1 mmol/L during days 8–29 were significantly higher with SZC 5 and 10 g once daily than placebo in HARMONIZE [15] (Table 1). Moreover, in this study, the mean number of normokalaemic days during days 8–29 was significantly (p < 0.001) higher with SZC 5 and 10 g once daily than placebo (13.4 and 13.9 vs. 7.4 days), with the median time to the first hyperkalaemic measure being significantly (p ≤ 0.002) longer with SZC 5 and 10 g once daily than placebo (14 and 28 vs. 7 days) [15]. In ZS-003, the mean number of normokalaemic days during the 12-day maintenance period was significantly higher with SZC 10 g once daily (n = 63) compared with placebo (n = 61) [10.2 vs. 8.2 days; p = 0.005] [8].
In the studied populations, therapy with SZC maintained serum potassium levels within the normal range regardless of the underlying cause of hyperkalaemia, age, sex, race, comorbid disease or concomitant use of RAAS inhibitor therapy [8]. For instance, in 87 patients with hyperkalaemia and heart failure who had achieved normokalaemia during the first 48 h of HARMONIZE, SZC 5 and 10 g once daily maintained significantly lower mean serum potassium levels throughout the 28-day maintenance period than placebo (4.7 and 4.5 vs. 5.2 mmol/L; p < 0.001) despite RAAS inhibitor doses being kept constant [17]. Moreover, significantly (p < 0.01) greater proportions of SZC 5 and 10 g once daily recipients than placebo recipients maintained normokalaemia during days 8–29 of the maintenance period (83 and 89 vs. 40%) [17].
Upon the discontinuation of SZC in ZS-003, mean serum potassium levels increased to near baseline levels within 7 days (no data reported) [8, 14].
3.2 ZS-004E and ZS-005
Patients who discontinued (because of hypokalemia or persistent hyperkalaemia) or completed the maintenance period of HARMONIZE were eligible to enter ZS-004E [9, 19]. Those with a potassium level of 3.5–5.5 mmol/L at the end of HARMONIZE directly entered the maintenance period of ZS-004E and received a starting SZC dosage of 10 g once daily. Those with a potassium level of > 5.5 mmol/L at the end of HARMONIZE received SZC 10 g three times daily for 24 or 48 h; if the potassium level had been lowered to 3.5–5.0 mmol/L during this time period, patients then entered the ZS-004E maintenance period and received a starting dosage of 10 g once daily [19]. During the up to 11-month maintenance period, the SZC dosage could be adjusted based on efficacy. Specifically, patients receiving SZC 10 g once daily could have their dosage of SZC increased to 15 g once daily (if their potassium level increased to > 5.5 mmol/L) or decreased to 5 g once daily (if their potassium level decreased to 3.0–3.4 mmol/L). Patients receiving SZC 5 g once daily could have their dosage of SZC decreased to 5 g once every other day (if their potassium level decreased to 3.0–3.4 mmol/L) [19].
Preliminary data from ZS-004E (n = 123) showed that serum potassium levels were maintained within the normal range with longer-term SZC therapy [8]. At 11 months, the average serum potassium level was 4.7 mmol/L, and 88% of patients had an average serum potassium level of < 5.1 mmol/L; 93, 77 and < 1% of the serum potassium measurements were between 3.5 to 5.5 mmol/L, between 3.5 and 5.1 mmol/L and < 3.5 mmol/L, respectively [8]. Upon the discontinuation of SZC in ZS-004E, mean serum potassium levels slowly increased (no data reported) [8, 9].
ZS-005 evaluated the efficacy of SZC over ≤ 12 months in 751 adult outpatients with a serum potassium level of ≥ 5.1 mmol/L (data from abstracts [20,21,22,23,24,25] and ClinicalTrials.gov [26]). Patients received SZC 10 g three times daily for 24–72 h [20, 26]. Those achieving a potassium level of 3.5–5.0 mmol/L during this time period (n = 746) then entered the maintenance period and received a starting dosage of SZC 5 g once daily. During the ≤ 12-month maintenance period, the SZC dosage could be adjusted based on efficacy. Specifically, patients receiving SZC 5 g once daily or 5 g once every other day who had a potassium level of > 5.0 mmol/L and those receiving SZC 10 g once daily who had a potassium level of > 5.5 mmol/L could have their dosage of SZC increased in 5 g once daily increments to a maximum dosage of 15 g once daily. Patients with a potassium level of 3.0–3.4 mmol/L could have their SZC dosage reduced in 5 g once daily decrements to a minimum dosage of 5 g once every other day, with those patients receiving SZC 5 g once every other day who had a potassium level of 3.0–3.4 mmol/L withdrawn from the study [20, 26]. There was no limit to the number of dose titrations permitted [26]. The mean daily dose of SZC during the maintenance period was 7.2 g, and 466 patients completed the period [20]. Overall, 74, 70 and 38% of patients had an eGFR of < 60 mL/min/1.73 m2, were receiving RAAS inhibitors or had heart failure, respectively [20]. Of note, heart failure was defined as in ZS-003 and HARMONIZE [16] (see Sect. 3.1).
SZC was effective in lowering serum potassium levels to within the normal range during the correction period, with 78% of patients achieving normokalaemia (primary endpoint; measured by central laboratory) and mean serum potassium levels reduced from 5.6 mmol/L (baseline value) to 4.8 mmol/L [20]. Moreover, the treatment effect of SZC on serum potassium levels was maintained during the ≤ 12-month maintenance period, with mean serum potassium levels of ≤ 5.1 mmol/L during months 3–12 (primary endpoint) achieved by 88% of patients [20] and mean serum potassium levels during months 3–12, 6–9 and 9–12 of 4.7, 4.7 and 4.6 mmol/L, respectively [26]. Upon the discontinuation of SZC in ZS-005, a small increase in mean serum potassium levels was seen within 7 days (no data reported) [20].
In a post hoc analysis of week 1, month 1 and month 3 data, mean serum potassium levels were 4.8, 4.8 and 4.7 mmol/L, respectively [21]. Moreover, additional post hoc analyses found that SZC therapy was effective in lowering serum potassium levels to within the normal range and maintaining normokalaemia regardless of eGFR [22], diabetes [23] or the concomitant use of RAAS inhibitor therapy [24]. SZC 10 g three times daily was also shown to be effective in lowering serum potassium levels to within the normal range in patients with a baseline serum potassium level of ≥ 6.0 mmol/L [post hoc subgroup analysis (n = 126)] [25].
4 Tolerability and Safety of SZC
SZC was generally well tolerated over ≤ 12 months in adults with hyperkalaemia participating in the phase III clinical studies discussed in Sect. 3. In a pooled analysis of data from phase II and III studies in 1760 patients with hyperkalaemia (including 430 who were exposed for 1 year), the most commonly reported (≥ 1/100 to < 1/10) adverse reactions across the SZC dosages were hypokalemia and oedema-related events [8]. Oedema-related events were only observed in the maintenance period and were thought to be dose dependent, with such events seen more frequently in patients receiving the 15 g once daily dosage [8].
In the initial 48 h of treatment, the incidence of treatment-emergent AEs (TEAEs) was generally similar in the SZC 10 g three times daily and placebo groups in ZS-003 (occurring in 11.9% of 143 and 10.8% of 158 patients) [14], and was 7.8% with SZC 10 g three times daily (n = 258) in HARMONIZE [15]. Treatment-related AEs (TRAEs) in HARMONIZE occurred in 2.3% of SZC 10 g three times daily recipients [15]. Constipation was the most frequently reported TEAE with this SZC dosage both in ZS-003 (2.1 vs. 0.6% of placebo recipients) [14] and HARMONIZE (0.8%) [15], although diarrhoea was the most common TRAE where specified (1.2% of recipients) [15]. Across the two studies [14, 15], hypokalemia was reported in one patient (< 1%) receiving SZC 10 g three times daily for 48 h; it was mild (serum potassium level of 3.4 mmol/L), transient and resolved without potassium repletion [14].
In the (12-day [14] or 28-day [15]) maintenance periods of ZS-003 and HARMONIZE, the tolerability profile of SZC 5 and 10 g once daily was generally comparable with that of placebo [14, 15]. TEAEs occurred in 21.5% of 65 patients in the SZC 5 g once daily group versus 23.5% of 68 patients in the corresponding placebo group and 33.3% of 63 patients in the SZC 10 g once daily group versus 24.6% of 61 patients in the corresponding placebo group in ZS-003 [14], and in 53.3% of 45 SZC 5 g once daily recipients, 29.4% of 51 SZC 10 g once daily recipients and 31.8% of 85 placebo recipients in HARMONIZE [15]. However, where specified [15], fewer than 7.0% of patients experienced TRAEs with SZC 5 g once daily or SZC 10 g once daily (6.7 and 5.9 vs. 8.2% of placebo recipients). No serious TRAEs with SZC therapy were reported in ZS-003 and HARMONIZE [14, 15]. One patient in each study died, but neither death was considered to be related to the study medication [12, 14, 15].
The most frequently reported TEAEs (occurring with a numerically higher incidence in the SZC group than the placebo group) were vomiting (4.6 vs. 0% of patients) [14] and upper respiratory tract infection (6.7 vs. 1.2%) [15] with SZC 5 g once daily and constipation (4.8 vs. 0%) [14] and oedema (5.9 vs. 2.4%) [15] with SZC 10 g once daily. Of note, constipation was not reported in the SZC 5 g once daily group and its corresponding placebo groups in ZS-003 [14] and was observed in numerically fewer SZC 5 and 10 g once daily recipients than placebo recipients in HARMONIZE (0, 2.0 and 7.1%) [15]. Moreover, the incidence of oedema in SZC 5 g once daily recipients in HARMONIZE was generally similar to that seen in placebo recipients (2.2 vs. 2.4%), with changes to therapy not required by any of the patients receiving SZC who developed oedema [15].
In HARMONIZE, there were no cases of hypokalemia reported as an AE; serum potassium levels < 3.5 mmol/L were described in 0, 9.8 and 0% of SZC 5 g once daily recipients, SZC 10 g once daily recipients and placebo recipients, respectively [15]. All cases were mild (3.0–3.4 mmol/L) and resolved upon reduction of the study medication dose [15]. The incidence of clinically relevant arrhythmia did not differ between the SZC and placebo groups in ZS-003 [14], with no clinically relevant arrhythmias observed in HARMONIZE [15].
Serum bicarbonate levels were increased in both the correction (48 h) and maintenance (≤ 28-day) periods with SZC therapy, but values in both treatment periods remained within the normal range [27]. In a pooled analysis [27] of ZS-003 [14] and HARMONIZE [15], the mean change from baseline in serum bicarbonate levels was 1.6 and − 0.2 mmol/L with SZC 10 g three time daily (n = 425) and placebo (n = 188) during the correction period and 1.1, 2.3 and 0.6 mmol/L with SZC 5 and 10 g once daily and placebo (n = 110, 114 and 301), respectively, in the maintenance period. Moreover, no clinically meaningful changes were observed in other clinical chemistry parameters (e.g. calcium, magnesium, sodium) or vital signs (e.g. blood pressure, bodyweight) [14, 15, 27].
The safety profile of SZC in patients with hyperkalaemia and heart failure was generally consistent with that seen in the overall population of HARMONIZE [17]. TEAEs were reported in 10.6% of the 94 patients who received SZC 10 g three times daily for 48 h, with nausea and dizziness the most frequently reported TEAEs (with each occurring in 2.1% of patients) [17]. During the maintenance period, TEAEs occurred in 55.6, 38.9 and 34.6% of the patients receiving SZC 5 g once daily, SZC 10 g once daily and placebo (n = 18, 18 and 26, respectively), with oedema the most frequently reported TEAE (occurring in one, two and one patients). No clinically relevant cases of hypokalemia (serum potassium level of < 3.0 mmol/L) or cardiac arrhythmia occurred in this patient subgroup, although one patient in the 10 g once daily subgroup developed mild hypokalemia (serum potassium level of 3.0 to < 3.5 mmol/L), which resolved following dose adjustments. There were no serious TRAEs with SZC therapy in patients with hyperkalaemia and heart failure [17].
Preliminary data from ZS-004E (n = 123) [12] and ZS-005 (n = 746) [20] suggest that the safety profile of SZC remains consistent over the longer term (≤ 12 months). TEAEs were reported in 66.7% and 65.5% of patients in the respective studies [12, 20]. Oedema-related events occurred in 16 (13%) patients in ZS-004E, with 11 of these 16 patients determined to be at a substantial increased risk for fluid overload-related events [although whether these patients were predisposed to an increased risk of fluid overload (e.g. had severe heart failure or renal insufficiency) was not reported)] [12]. In ZS-005, anaemia, constipation, hypertension, peripheral oedema and urinary tract infection each occurred in > 5% of patients, and laboratory-determined hypokalemia occurred in 5.8% of patients (with 1.2% of patients having a potassium level of 2.5 to < 3.0 mmol/L) [20]. Where reported [12], 11.4% of patients experienced a TRAE, with the most frequently reported (in three patients) being muscle spasms. Serious TEAEs occurred in 19.5% of patients (none of which were considered by the investigators to be related to the study medication) in ZS-004E [12] and 21.6% of patients in ZS-005 [20]. No patients died in ZS-004E [12]; in ZS-005, eight (1.1%) patients died [20]. No clinically significant mean changes from the baseline of HARMONIZE in blood pressure, heart rate, respiration rate, weight or temperature were observed in ZS004E [27].
The safety profile of SZC across various patient subgroups (i.e. baseline serum potassium level of ≥ 6.0 mmol/L; concomitant use of RAAS inhibitor therapy; diabetes; eGFR < 30 mL/min/1.73 m2) was generally consistent with that seen in the overall population, according to post hoc subgroup analyses of ZS-005 [22,23,24,25].
5 Dosage and Administration of SZC
SZC, available as a powder for oral suspension (in water), is approved in the EU for the treatment of hyperkalaemia in adults [8]. The recommended starting dosage is 10 g three times daily, with normokalaemia typically achieved within 24–48 h. This regimen can be continued for a further 24 h in patients still hyperkalaemic following 48 h of therapy, but other treatment approaches should be considered if normokalaemia is not achieved after 72 h of treatment. Upon achieving normokalaemia, the recommended starting dosage for maintenance is 5 g once daily, with the dosage titrated up to a maximum of 10 g once daily or down to a minimum of 5 g once every other day as required to maintain a potassium level within the normal range. SZC can be taken with or without food [8]. Local prescribing information should be consulted for detailed information regarding reconstitution and administration, missed doses, contraindications, use in special patient populations, and warnings and precautions.
6 Place of SZC in the Management of Hyperkalaemia
Serum potassium levels above the normal range (i.e. hyperkalaemia) reduce the large potassium gradient that regulates the resting membrane potential (Sect. 1), thereby affecting voltage-dependent processes in excitable (e.g. muscle and nerve) cells [2, 3, 6]. The most concerning effects are on cardiac cells, with cell membrane depolarization reducing cardiac excitability and the action potential duration, and slowing conduction, leading to bradyarrhythmias, ventricular fibrillation and asystole [2, 3, 6, 7].
The UK clinical practice guidelines on the treatment of acute hyperkalaemia in adults [4] and a recent scientific workshop (cosponsored by the National Kidney Foundation and the American Society of Hypertension) report on potassium homeostasis in health and disease [7] both recommend the use of calcium (to stabilize cardiac conduction) and glucose, insulin and a β2-adrenergic receptor agonist (e.g. salbutamol) [to redistribute extracellular potassium to the intracellular space] for the acute management of hyperkalaemia, with the scientific workshop report also recommending sodium bicarbonate as an option for patients with concurrent metabolic acidosis [7]. To encourage potassium excretion, the use of cation-exchange resins is recommended in the UK clinical practice guidelines [4] for the management of acute mild to moderate hyperkalaemia, and the use of potassium-binding agents or potassium-wasting diuretics is recommended in the scientific workshop report for the long-term management of hyperkalaemia [7]. However, such methods are reported to be used less frequently than a dose reduction in or the discontinuation of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and potassium-sparing diuretics [7].
The cation-exchange resins SPS and calcium polystyrene sulfonate (CPS) exchange sodium or calcium, respectively, for various cations, including potassium [2, 4]. However, their onset of action is slow (e.g. ≥ 2 h for SPS [6]) and their use is associated with serious gastrointestinal AEs (e.g. intestinal necrosis) [2, 4]. Moreover, SPS should be used cautiously in patients who are unable to tolerate even small increases in sodium levels (such as those with hypertension, oedema or severe congestive heart failure) [2]. Recently, two potassium-binding agents (SZC and patiromer) have been developed [7]. Both are insoluble, non-absorbed powders for oral suspension; however, SZC is a non-polymer and exchanges hydrogen and sodium for potassium and ammonium ions in the GIT (Sect. 2), while patiromer is a polymer (and uses sorbitol as a cathartic) and exchanges calcium for potassium in the GIT [7, 9, 28]. SZC has a > 25-fold higher selectivity for potassium ions over calcium and magnesium ions in vitro, with the exchange capacities of SZC for calcium and magnesium ions being below the LLOQ (Sect. 2). This is reflected clinically, with SZC not affecting serum calcium or magnesium levels in patients with hyperkalaemia (Sects. 2 and 4). In contrast, patiromer has been associated with low serum magnesium levels in clinical studies [28]. SZC does exchange sodium (and hydrogen) for potassium (and ammonium), which is of theoretical concern in patients in whom oedema and volume overload could worsen their clinical condition [7]. However, in adults with hyperkalaemia receiving SZC, no clinically meaningful changes in serum sodium levels have been seen and serum bicarbonate levels, albeit increased, remained within the normal range (Sect. 4). SZC and patiromer also differ in terms of their onset of action (1 h for SZC (Sect. 3.1.1) and 4–7 h for patiromer [28]), suggesting the former may be suitable for the acute management of hyperkalaemia.
Results from multinational, phase III studies have demonstrated the serum potassium-lowering efficacy of SZC in adults with hyperkalaemia (Sect. 3). In ZS-003 and HARMONIZE, SZC 10 g three times daily lowered serum potassium levels to within the normal range (3.5–5.0 mmol/L) during the first 48 h of treatment, with significant mean reductions from baseline seen 1 h after the first dose and at all subsequent timepoints during the 48-h period (Sect. 3.1.1). Of note, patients with higher baseline serum potassium levels demonstrated a greater response to SZC therapy than those with lower baseline serum potassium levels (Sect. 3.1.1). Serum potassium levels were maintained within the normal range with SZC 5 and 10 g once daily for ≤ 28 days in these studies (Sect. 3.1.2).
The beneficial effects of therapy with SZC 10 g three times daily for the first 48 h (Sect. 3.1.1) and with SZC 5 and 10 g once daily for ≤ 28 days (Sect. 3.1.2) were consistent across all patient subgroups, including comorbid disease (e.g. CKD and heart failure) and the concomitant use of RAAS inhibitor therapy. It is worth noting that patients undergoing dialysis, those with diabetic ketoacidosis or those with cardiac arrhythmias requiring immediate treatment were among those excluded from ZS-003 and HARMONIZE (Sect. 3.1). Studies investigating the efficacy of SZC in these and other patient groups (e.g. hospitalized patients and patients with serum potassium levels of > 6.5 mmol/L) would be of interest, as would studies directly comparing SZC with other potassium-binding agents (e.g. patiromer).
SZC was generally well tolerated in adults with hyperkalaemia participating in ZS-003 and HARMONIZE (Sect. 4). Its tolerability profile was generally similar to that seen with placebo in the 48-h correction period of ZS-003 and in the ≤ 28-day maintenance period of both studies, and its safety profile across various patient subgroups was consistent with that seen in the overall populations in these studies (Sect. 4). The most frequently reported AE varied across these studies, but was mostly gastrointestinal in nature, and the incidence of individual AEs (occurring with a numerically higher incidence in the SZC group than the placebo group) was low (≤ 6.7%). No serious TRAEs were reported in ZS-003 and HARMONIZE.
Preliminary data from the as yet to be published ZS-004E and ZS-005 studies showed that the treatment effect of SZC on serum potassium levels was maintained (Sect. 3.2) and the safety profile of SZC appeared to remain consistent (Sect. 4) over the longer term (≤ 12 months). No serious TRAEs were reported (Sect. 4). Further data from these studies are awaited with interest, as are long-term (> 12 months) data.
In conclusion, although data from longer-term studies are not yet fully available, current evidence indicates that SZC is a promising therapy for the management of hyperkalaemia in adults.
Data Selection Sodium Zirconium Cyclosilicate: 181 records identified
Duplicates removed | 50 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 30 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 73 |
Cited efficacy/tolerability articles | 11 |
Cited articles not efficacy/tolerability | 16 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were sodium zirconium cyclosilicate, ZS-9, Lokelma, hyperkalaemia. Records were limited to those in English language. Searches last updated 8 October 2018 | |
Change history
20 March 2019
The article Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia, written by Sheridan M. Hoy, was originally published Online First without open access.
20 March 2019
The article Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia, written by Sheridan M. Hoy, was originally published Online First without open access.
20 March 2019
The article Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia, written by Sheridan M. Hoy, was originally published Online First without open access.
20 March 2019
The article Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia, written by Sheridan M. Hoy, was originally published Online First without open access.
References
Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med. 2015;373(1):60–72.
Tamargo J, Caballero R, Delpón E. New therapeutic approaches for the treatment of hyperkalaemia in patients treated with renin-angiotensin-aldosterone system inhibitors. Cardiovasc Drugs Ther. 2018;32(1):99–119.
Dixon BS. Zirconium cyclosilicate for treatment of hyperkalaemia. JAMA. 2014;312(21):2217–8.
UK Renal Association. Clinical practice guidelines: treatment of acute hyperkalaemia in adults. 2014. http://www.renal.org. Accessed 3 Aug 2018.
Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018;93(2):325–34.
Long B, Warix JR, Koyfman A. Controversies in management of hyperkalaemia. J Emerg Med. 2018;55(2):192–205.
Kovesdy CP, Appel LJ, Grams ME, et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis. 2017;70(6):844–58.
AstraZeneca. Lokelma (sodium zirconium cyclosilicate) for oral suspension: EU summary of product characteristics. 2018. http://www.ema.europa.eu/ema/. Accessed 3 Aug 2018.
AstraZeneca. Lokelma™ (sodium zirconium cyclosilicate) for oral suspension: US prescribing information. 2018. http://www.fda.gov/. Accessed 3 Aug 2018.
Stavros F, Yang A, Leon A, et al. Characterization of structure and function of ZS-9, a K + selective ion trap. PLoS One. 2014;9(12):e114686.
Zannad F, Rasmussen HS, Lavin PT, et al. Effect of sodium zirconium cyclosilicate (ZS-9) on aldosterone from the phase 3 randomized, double-blind, placebo-controlled HARMONIZE study [abstract no. P1592]. Eur J Heart Fail. 2015;17(Suppl.):342.
European Medicines Agency. Sodium zirconium cyclosilicate: EU assessment report. 2018. http://www.ema.europa.eu/ema/. Accessed 3 Aug 2018.
Ash SR, Singh B, Lavin PT, et al. A phase 2 study on the treatment of hyperkalaemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–11.
Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalaemia. N Engl J Med. 2015;372(3):222–31.
Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalaemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–33.
Data on file, AstraZeneca, 2018.
Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17(10):1050–6.
Kosiborod M, McCullough PA, Rasmussen H, et al. Acute efficacy of sodium zirconium cyclosilicate (ZS-9) in patients on mineralocorticoid-receptor antagonists: analysis from two phase 3 studies. Circulation 2015;132(Suppl 3).
US National Institutes of Health. ClinicalTrials.gov identifier NCT02107092. 2016. http://clinicaltrials.gov/. Accessed 3 Aug 2018.
Fishbane S, Adler S, Singh B, et al. Maintained efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: 12-month, open-label, phase 3 study [abstract no. TH-PO1112]. J Am Soc Nephrol. 2017;28(Suppl):390.
Spinowitz BS, Pergola PE, Lerma EV, et al. Initial response rates for sodium zirconium cyclosilicate treatment of hyperkalaemia [abstract no. 281]. Am J Kidney Dis. 2018;71(4):585–6.
Roger S, Lavin P, Lemma E, et al. Safety and efficacy of sodium zirconium cyclosilicate for long-term treatment of hyperkalaemia in patients with chronic kidney disease: results from an open-label, phase 3 study [abstract no. FP071]. Nephrol Dial Transplant. 2018;33(Suppl 1):i72.
Fishbane S, Roger S, Packham D, et al. Sodium zirconium cyclosilicate for hyperkalaemia in patients with diabetes mellitus: retrospective analysis of a 12 month open label, phase 3 study [abstract no. SP421]. Nephrol Dial Transplant. 2018;33(Suppl 1):i489–90.
McCullough P, Pergola P, Fishbane S, et al. Efficacy and safety of sodium zirconium cyclosilicate to treat hyperkalaemia among patients taking renin-angiotensin-aldosterone system inhibitors in a 12-month open-label, phase 3 study: a post hoc subgroup analysis [abstract no. 16610]. Circulation. 2017;136(Suppl 1):A16610.
Packham D, Roger S, Pergola P, et al. Acute efficacy of sodium zirconium cyclosilicate for hyperkalaemia in outpatients with potassium ≥ 6.0 mEq/L: post-hoc subgroup analysis of a phase 3 trial [abstract no. TH-PO1113]. J Am Soc Nephrol. 2017;28(Suppl):390.
US National Institutes of Health. ClinicalTrials.gov identifier NCT02163499. 2018. http://clinicaltrials.gov/. Accessed 3 Aug 2018.
US FDA Center for Drug Evaluation and Research. Medical review(s). 2017. http://www.fda.gov/. Accessed 3 Aug 2018.
Vifor France. Veltassa (patiromer): EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 3 Aug 2018.
Acknowledgements
During the peer review process, the manufacturer of sodium zirconium cyclosilicate was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Sheridan Hoy is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: P. C. Deedwania, UCSF School of Medicine, San Francisco, CA, USA; S. D. Roger, Department of Renal Medicine, Gosford Hospital, Gosford, NSW, Australia.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Hoy, S.M. Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia. Drugs 78, 1605–1613 (2018). https://doi.org/10.1007/s40265-018-0991-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0991-6