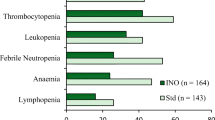
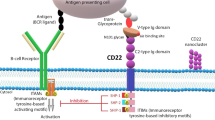
Abstract
Intravenous inotuzumab ozogamicin (Besponsa®; Pfizer) is an anti-CD22 monoclonal antibody-calicheamicin conjugate that binds to CD22-expressing tumour cells. Upon binding, the complex is internalised and the cytotoxic calicheamicin derivative is released inside the cell, inducing double-strand DNA breakage and subsequent cell death. In June 2017, the EMA granted inotuzumab ozogamicin approval as monotherapy for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukaemia (ALL). The use of inotuzumab ozogamicin in adult patients with Philadelphia chromosome-positive, relapsed or refractory CD22-positive B-cell precursor ALL is restricted to those who have failed treatment with at least one tyrosine kinase inhibitor. Inotuzumab ozogamicin was granted priority review for the treatment of relapsed or refractory B-cell precursor ALL by the US FDA in February 2017. In the USA, a phase III trial evaluating inotuzumab ozogamicin in combination with frontline chemotherapy in adults with newly diagnosed B-cell ALL has recently been initiated and inotuzumab ozogamicin is under phase II evaluation in childhood CD22-positive B-cell ALL. Inotuzumab ozogamicin combination therapies are also being evaluated in the phase I/II or II setting in ALL and chronic myeloid leukaemia and in the phase I setting in Burkitt’s lymphoma. This article summarises the milestones in the development of inotuzumab ozogamicin leading to this first approval for ALL.
Similar content being viewed by others
References
Thota S, Advani A. Inotuzumab ozogamicin in relapsed B-cell acute lymphoblastic leukemia. Eur J Haematol. 2017;98(5):425–34.
Yilmaz M, Richard S, Jabbour E. The clinical potential of inotuzumab ozogamicin in relapsed and refractory acute lymphocytic leukemia. Ther Adv Hematol. 2015;6(5):253–61.
Pfizer. Besponsa approved in the EU for adult patients with relapsed or refractory B-cell percursor acute lymphoblastic leukemia [media release]. 30 Jun 2017. http://www.pfizer.com/.
Pfizer Limited. Besponsa (inotuzumab ozogamicin) 1 mg powder for concentrate for solution for infusion: summary of product characteristics. 2017. Accessed 21 Jul 2017.
Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Wagner-Johnston ND, Goy A, Rodriguez MA, et al. A phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplant in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(10):2863–9.
Goy A, Forero A, Wagner-Johnston N, et al. A phase 2 study of inotuzumab ozogamicin in patients with indolent B-cell non-Hodgkin lymphoma refractory to rituximab alone, rituximab and chemotherapy, or radioimmunotherapy. Br J Haematol. 2016;174(4):571–81.
European Medicines Agency. Summary of opinion (initial authorisation): Besponsa (inotuzumab ozogamicin). 2017. http://www.ema.europa.eu/. Accessed 21 Jul 2017.
Pfizer. Pfizer announces acceptance of regulatory submission for inotuzumab ozogamicin by the U.S. Food and Drug Administration [media release]. 21 Feb 2017. http://www.pfizer.com/.
Celltech Group Plc. Celltech Group plc oncology pipeline update [media release]. 16 Jun 2003. http://www.celltechgroup.com/.
Pfizer, Wyeth. Pfizer completes acquisition of Wyeth [media release]. 16 Oct 2009. http://www.pfizer.com/.
DiJoseph JF, Dougher MM, Evans DY, et al. Preclinical anti-tumor activity of antibody-targeted chemotherapy with CMC-544 (inotuzumab ozogamicin), a CD22-specific immunoconjugate of calicheamicin, compared with non-targeted combination chemotherapy with CVP or CHOP. Cancer Chemother Pharmacol. 2011;67(4):741–9.
DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807–14.
de Vries JF, Zwaan CM, De Bie M, et al. The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia. 2012;26(2):255–64.
DiJoseph JF, Dougher MM, Armellino DC, et al. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5.
DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017;1(15):1167–80.
Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4(8):e387–98.
Garrett M, Ruiz-Garcia A, Parivar K, et al. Characterization of inotuzumab ozogamicin time-dependent clearance in relapsed/refractory acute lymphoblastic leukemia patients by nonlinear mixed-effects analysis [abstract no. PC-26]. Clin Pharmacol Ther. 2016;99(Suppl 1):S11.
Kebriaei P, DeAngelo DJ, Stelljes M, et al. Factors associated with allogeneic hematopoietic stem cell transplantation (HSCT) outcomes in patients (pts) with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treated with inotuzumab ozogamicin (InO) versus (v) conventional chemotherapy (C) [abstract no. 7007]. J Clin Oncol. 2017;35(15 Suppl):7007.
Kantarjian HM, Su Y, Jabbour EJ, et al. Patient-reported outcomes from a global phase 3 randomized controlled trial of inotuzumab ozogamicin versus standard of care chemotherapy for relapsed/refractory acute lymphoblastic leukemia [abstract no. 1599]. Blood. 2016;128(22):1599.
van Oostrum I, Su Y, Heeg B, et al. Quality-adjusted life years (QALY) for inotuzumab ozogamicin vs standard of care for relapsed/refractory acute lymphoblastic leukemia (R/R ALL) [abstract no. e18506]. J Clin Oncol. 2017;35(15 Suppl).
Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–11.
Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–36.
Rytting M, Triche L, Thomas D, et al. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(2):369–72.
Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) [abstract no. 10512]. J Clin Oncol. 2017;35(15 Suppl):10512.
Assi R, Kantarjian HM, Ravandi F, et al. Inotuzumab ozogamicin (IO) combined with mini-hyper-CVD as salvage therapy for patients (pts) with R/R acute lymphoblastic leukemia (ALL) [abstract no. 7025]. J Clin Oncol. 2017;35(15 Suppl):7025.
Sasaki K, Jabbour EJ, O’Brien SM, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-hyper-CVD) as frontline therapy for older patients with acute lymphoblastic leukemia (ALL): interim result of a phase II clinical trial [abstract no. 588]. Blood. 2016;128(22):588.
Advani AS, Moseley A, Liedtke M, et al. A phase 1 trial of inotuzumab in combination with CVP (cyclophosphamide, vincristine, prednisone) for relapsed/refractory CD22+ acute leukemia (SWOG 1312) [abstract no. 1634]. Blood. 2016;128(22):1634.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/81E8F0601F5B01D6.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Inotuzumab Ozogamicin: First Global Approval. Drugs 77, 1603–1610 (2017). https://doi.org/10.1007/s40265-017-0802-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0802-5