Abstract
Introduction
Reducing the occurrence of drug-related problems is a global health concern. In mental health hospitals, drug-related problems are common, leading to patient harm, and therefore understanding their potential risk factors is key for guiding future interventions designed to minimise their frequency.
Objective
The aim of this systematic review was to explore the potential risk factors of drug-related problems in mental health inpatient units.
Methods
Six databases were searched between 2000 and 2021 to identify studies that investigated the potential risk factors of drug-related problems in adults hospitalised in mental health inpatient units. Data extraction was performed by two authors independently and Allan and Barker’s criteria were used for study quality assessment. Studies were categorised based on drug-related problem types and potential risk factors were stratified as patient, medication, and hospital related.
Results
A total of 22 studies were included. Studies mostly originated in Europe (n = 19/22, 86.4%), and used a multivariable logistic regression to identify potential risk factors (n = 13, 59%). Frequently investigated factors were patient age (n = 14/22), sex (n = 14/22) and the number of prescribed medications (n = 14/22). Of these, increasing the number of prescribed medications was the only factor consistently reported to be significantly associated with the occurrence of most types of drug-related problems (n = 11/14).
Conclusions
A variety of patient, medication and hospital-related potential risk factors of drug-related problems in mental health inpatient units were identified. These factors could guide the development of interventions to reduce drug-related problems such as pharmaceutical screening tools to identify high-risk patients for timely interventions. Future studies could test a wider range of possible factors associated with drug-related problems using standardised approaches.
Clinical Trial Registration
PROSPERO: CRD42021279946.
Similar content being viewed by others
Greater numbers of prescribed medications are associated with an increased risk of drug-related problems in hospital-based mental health inpatients units. |
There is a lack of evidence on a wider range of potential risk factors of drug-related problems in mental health inpatient units. |
Identified potential risk factors in this review could assist in developing interventions to reduce drug-related problems such as pharmaceutical prioritisation tools for use in mental health inpatient units. |
1 Introduction
A drug-related problem (DRP) is defined as “An event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” [1]. Drug-related problems are highly prevalent in mental healthcare with one study reporting a prevalence of 21.3% of which 17.21% were medication errors (MEs) and 4.12% were adverse drug events (ADEs) [2]. A systematic review published in 2017 reported the rates of MEs and ADEs in psychiatric hospitals to be 10.6–17.5 and 10–42 per 1000 patient-days, respectively [3]. In a study published in 2021 originating from England, ADEs were reported to affect 12.6% of patients in mental health hospitals [4]. As DRPs may lead to patient morbidity and mortality [5], the World Health Organization (WHO) has launched its third Global Patient Safety Challenge on Medication Safety with a goal to reduce the incidence of preventable medication harm by half within 5 years [6]. To reach this goal and improve patient safety, data on the risk factors of DRPs are of utmost importance to inform the development of interventions.
Risk factors are warning signs that, when identified by healthcare professionals, alert them and direct their attention to areas where it is most needed. Understanding the potential risk factors (PRFs) of DRPs is important as this information may help healthcare professionals identify and prevent DRPs [7]. Such factors, when identified, can be incorporated into interventions to reduce the frequency of DRPs. Predictive models can be developed based on a set of risk factors to identify people at high risk of a particular condition and offer them a timely intervention [8]. Potential risk factors for DRPs can also be used to help pharmacists identify patients in most need of medication reviews through the development of pharmaceutical care prioritisation tools [9].
Whilst there are a number of published reviews of DRPs in mental healthcare [3, 10,11,12,13], most of these focused on the prevalence and types of DRPs with only one examining PRFs of DRPs [12]. This review, however, was restricted to the older patient population and was non-systematic in nature. Hence, despite existing evidence on the prevalence and types of DRPs in mental health, there is a lack of data on their risk factors.
Although there are some systematic reviews of PRFs of DRPs in general hospitals [14,15,16], none focused on mental health settings where PRFs may differ. For example, patients in mental health hospitals may have cognitive impairment that affects their medication use [17] and may lead to different patient-related PRFs. Psychotropic medications are commonly used long term [18] and at high doses [19], whereas parenteral medications are seldom used in psychiatry units [20], which may lead to different medication-related PRFs. Last, hospital factors also differ as patients in mental health units might have their medications administered in a central location such as clinic rooms instead of having them in their beds as observed in general hospitals [21]. Whilst examples of studies that primarily evaluated the PRFs of DRPs exist in psychiatry [22], the literature on this topic is fragmented with some studies discussing the PRFs of DRPs in psychiatry as a secondary outcome [23]. Hence, a gap exists in our knowledge of PRFs of DRPs in mental healthcare and the nature of their association. Given the importance of this knowledge in developing interventions and limiting the frequency of DRPs, a review of PRFs of DRPs in mental health is warranted. We aim to comprehensively identify and characterise from the published literature the known PRFs of DRPs in hospital-based mental health units and to understand the type and extent of the relationship between each PRF and DRP.
2 Methods
The protocol for this review was prepared according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-P) guidelines [24] and was registered with PROSPERO (CRD42021279946).
2.1 Definitions
In this study, a ‘potential risk factor’ was defined as a correlate whose association with DRP occurrence was explored using formal statistical testing. This definition was derived from Offord and Kraemer who defined a correlate as “a variable that is associated, either positively or negatively, with an outcome” and considered a risk factor as a type of correlate that is associated with an increased probability of an outcome that is usually unpleasant [25].
Drug-related problems were defined as per the Pharmaceutical Care Network Europe [1] “A Drug-Related Problem is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes”. Drug-related problems in this study included adverse drug reactions (ADRs), ADEs, MEs, potentially inappropriate prescribing (PIP), and medication discrepancies (MDs). Definitions of DRP types can be seen in Table 1.
2.2 Search Strategy
A search strategy was developed using medical subject headings (MeSH) and related text words. The search strategy grid included four main keywords and their synonyms: drug related problems, psychiatry, risk factors, and hospital (see Electronic Supplementary Material [ESM]). All types of studies published between 1 January, 2000 and 10 September, 2021 were included with no language limits. The year 2000 was chosen to capture the modern healthcare era, as well as to coincide with the introduction of atypical antipsychotics and the publication of two landmark patient safety reports [32, 33]. Peer Review of Electronic Search Strategies (PRESS) [34] was used in developing the search strategy as its use was found to be beneficial in reducing errors and improving search strategies [34]. Moreover, the search strategy was reviewed by an external reviewer, a University of Manchester librarian.
2.3 Information Sources
The following databases were searched: MEDLINE, EMBASE, Web of Science, International Pharmaceutical Abstracts, PsycINFO, and CINAHL PLUS. The snowballing technique was used to find further related articles through relevant reviews and candidate studies [35].
2.4 Study Records
The results of the literature search were uploaded to Endnote [36] to remove duplicates. Afterwards, the results were uploaded to the Rayyan [37] application for systematic reviews to assist with the screening process.
The titles, abstracts and full texts of obtained records were screened to identify studies for inclusion in the review. Excluded reviews were screened for relevant references before they were eliminated. The screening process was conducted by FQ, but when eligibility was ambiguous, it was resolved through retaining the article for the next screening step and if necessary following discussion with all of the authors. When further information was needed for a particular study, study authors were contacted. There was no blinding of studies’ journals, authors or institutions.
Titles in other languages were translated using Google Translate whereas all identified abstracts were in English. Full-text non-English studies were translated using Google Translate to check their eligibility. If deemed eligible, articles were then translated by native speakers of the language who are fluent in English.
A data extraction form (see ESM) was developed and piloted across three included articles as standardised forms improve the validity and reliability of data extraction [38, 39]. The form was used to extract the following data: title, authors, country, year, demographics, aim and objectives of the study, study setting, study design, duration of the study, sample size, inclusion and exclusion criteria, DRP identification method, types of DRPs investigated, number of patients with DRPs, total number of DRPs, PRFs of DRPs and their strength of association, and statistical methods. The form underwent minor changes and clarifications before commencing full extraction by FQA. Data was also extracted independently for all included studies by two of the authors (PJL and RNK) to reduce the risk of errors [39, 40].
2.5 Inclusion and Exclusion Criteria
The inclusion and exclusion criteria are detailed in Table 2. A multivariable logistic regression might be an appropriate choice to test the association between PRFs and DRPs as it adjusts for potential confounders. There are, however, other tests that do not account for confounding factors, such as a Chi-square test and univariable regression. These tests are still useful in the absence of more robust methods in providing an insight, albeit limited, into possible predictors of DRPs. As the data on PRFs of DRPs in psychiatry are unknown, any formal statistical test was eligible for inclusion.
2.6 Quality Assessment
Quality assessment was carried out using Allan and Barker’s [41] criteria for ME studies. The criteria are made up of 13 questions (see ESM); however, 12 questions were applied as one focuses on a risk of bias irrelevant to the included studies.
2.7 Data Synthesis
Quantitative synthesis was not possible because of the heterogeneity of included studies and the different PRFs investigated in each study. Systematic descriptive synthesis for the collected data was carried out in accordance with the guidance from the Centre for Reviews and Dissemination [42]. Studies were categorised based on DRP types: PIP, MEs, ADRs, ADEs and MDs. If a study included more than one type of DRP without a separate analysis, it was categorised as a DRP. Potential risk factors for each type of DRP were grouped as patient, medication and hospital related.
3 Results
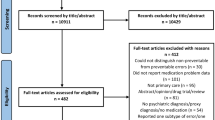
The database search retrieved 36,570 records (see ESM) of which 8708 were duplicates identified through Endnote (5405) and Rayyan (3303). A total of 27,862 records underwent screening followed by eligibility checking to identify 21 studies that met the inclusion criteria. Screening references of included studies identified one additional study, resulting in a final number of 22 studies included in the review. Details of the search results are demonstrated in a PRISMA chart (Fig. 1).
3.1 Characteristics of Included Studies
Of the included studies, eight identified PRFs of PIP [43,44,45,46,47,48,49,50], seven of MEs [20, 21, 51,52,53,54,55], three of ADRs [17, 50, 56], two of MDs [57, 58], one of ADEs [4], and two included a wider range of DRPs [59, 60]. Sixteen studies were conducted in specialised mental health institutions/hospital trusts [4, 20, 21, 45, 47,48,49, 51,52,53,54, 56,57,58,59,60] whereas six studies were completed in psychiatry units from general hospitals [17, 43, 44, 46, 50, 55]. The majority of the studies originated in Europe (19/22, 86.4%) [4, 17, 20, 21, 43,44,45,46,47,48,49,50,51,52,53,54,55, 58, 60], particularly in the UK (n = 7/21, 33.3%) [4, 20, 21, 52,53,54, 58]. The remaining studies were conducted in India (n = 2/21, 9.5%%) [56, 59] and the USA (n = 1/21, 4.8%) [57]. All studies were observational except for one that was interventional [44]. Thirteen studies used a multivariable logistic regression alone [47, 48, 54, 55, 58] or a multivariable logistic regression preceded by a univariate regression [4, 43,44,45,46, 50, 53] or bivariate regression [17] to test PRFs. Three studies were published between 2000 and 2010 [20, 51, 52] with 13 published between 2010 and 2020 [17, 21, 43,44,45,46, 53,54,55, 57,58,59,60] and six in 2021 [4, 47,48,49,50, 56]. Two non-English studies were included in the review [50, 51] and each was translated by a native speaker fluent in English. A summary of the study characteristics is presented in Table 3.
3.2 Quality Assessment
Only two studies reported all necessary research sections described by Allan and Barker [41] in question one [17, 54], including a clear and detailed introduction, methodology, results, interpretation and conclusion. None of the studies reported checking the assumptions for the statistical tests they performed. An approach to confirm validity of the DRP identification method was reported by the majority of studies (n = 15/22, 68%) while reliability was reported by eight studies (n = 8/22, 36.4%). The full quality assessment is presented in Table 4.
3.3 Potential Risk Factors of Potentially Inappropriate Prescribing
Eight studies investigated PRFs of PIP [43,44,45,46,47,48,49,50]. However, only two studies [44, 45] identified the PRFs of both PIMs and potentially inappropriate omissions whereas six studies [43, 46,47,48,49,50] identified PRFs of PIMs only. Of the eight studies, four [47,48,49,50] were carried out in Germany and used the German PRISCUS list [61] for PIMs and two [43, 44] were conducted in Switzerland and used the French adaptation of STOPP/START criteria [62] to identify both PIMs and potentially inappropriate omission. One study [46] used the French adaptation of Beers criteria [63, 64], and the final study [45] used the Beers criteria 2012 update [65] and the Dutch version of the STOPP/START criteria [66]. Four studies were prospective [43,44,45, 50] and four were retrospective [46,47,48,49]. Of these, only one was interventional [44]. All the studies used chart reviews to identify PIP and had a duration of 4 months or longer. Only two studies indicated that data collectors were trained [43, 44]. In one, data were collected by two trained physicians [43], and the other, which was interventional, by one blinded physician who was not involved in the intervention team [44]. Validity of the PIP identification method was reported by all the studies, and in one, the causal relationship was discussed in a case conference with a person specialised in clinical pharmacology or pharmacology and toxicology [50]. Multivariable logistic regression was used to identify DRPs in seven studies [43,44,45,46,47,48, 50], whereas chi-square testing was used in the remaining study [49].
Potential risk factors and protective factors identified for PIP are presented in Table 5a. An increasing number of medications was the most commonly reported PRF of PIMs [43, 45, 47, 48, 50] followed by an increased duration of hospitalisation [47, 49] and prior fall within the last 3 months [43, 44]. The Charlson Co-morbidity Index, which is a score calculated based on age and the presence and severity of 16 morbidities to predict mortality, was the most frequently reported PRF of PO [43,44,45]. Two protective factors were identified for PIMs in two studies, increased age [47, 50] and dementia diagnosis [50]. No protective factors were reported for potentially inappropriate omissions. An increased number of comorbidities was reported to be positively associated with the occurrence of PIMs in one study; however, it was borderline significant [adjusted odds ratio (AOR): 1.04 (95% confidence interval 1–1.06)] [49]. This same PRF was also found to be associated with a reduction in PIMs in another study, and again the association had borderline statistical significance [AOR: 0.97 (95% confidence interval 0.94–1.00)] [47].
3.4 Potential Risk Factors of Adverse Drug Events and Adverse Drug Reactions
The search revealed three studies that investigated PRFs of ADRs [17, 50, 56], and one for ADEs [4]. In one study [17] ADRs were defined as per WHO definition and were investigated upon admission and during hospitalisation. Another study [56] defined ADRs similarly. “The term “ADR” is used to describe the noxious or unintended reaction produced by the drug normally used in human. It can be subjective and objective and can be measured”. The third study [50] defined ADRs as “any unfavourable or undesired event that occurs during treatment with an active substance. An AE is temporally related to the administered drug. If there is also a causal relationship, this is referred to as an adverse drug reaction (ADR)” [67]. The study that investigated ADEs [4] defined them as “… any injury or harm related to the use of a drug” [68].
Only one study indicated the profession of data collectors [4], and they were clinical pharmacists who received training by the study authors and given a study manual that included training material. However, another study indicated that one geriatric medicine physician and one pharmacovigilance specialist reviewed the ADRs to ensure they were not related to the progression of the behavioural and psychological symptoms of dementia [17]. All the studies used chart reviews to collect the data and one study [4] utilised a trigger tool developed by the Institute for Healthcare Improvement [69]. All the studies used a multivariable logistic regression to identify PRFs except for one that used chi-square testing [56], and this study did not find any statistically significant PRF.
Identified PRFs significantly increasing the risk of ADRs included certain medication classes such as ACEIs/ARBs, anti-dementia agents, antidepressants, anti-diuretics, anti-arrhythmic, and neuroleptic medications (AOR = 2.07, 1.84, 1.64, 1.58, 2.21 and 2.04, respectively) [17]. Additionally, PIMs and an increased number of prescribed medications were reported to increase the risk of ADRs [50]. As for serious ADRs, reported PRFs included the use of ACEIs/ARBs, type 1, 3 and 4 anti-arrhythmic agents, and neuroleptic medication (AOR = 2.95, 2.71 and 2.42, respectively) [17]. Serious ADRs were also associated with the use of potentially inappropriate medications identified through the PRISCUS list [50].
The only ascertained PRF of the occurrence of ADEs was length of hospitalisation [4]. It was reported that patients hospitalised for eight to 30 days or >30 days had an increased risk of ADEs compared with patients hospitalised for 7 days or less. The full list of factors associated with ADRs and ADEs is presented in Table 5b.
3.5 Potential Risk Factors of Medication Errors
The PRFs of MEs were assessed by seven studies [20, 21, 51,52,53,54,55]. Of these, four examined prescribing errors (PEs) [52,53,54,55], two medication administration errors (MAEs) [20, 21], and one included other types of MEs [51]. It must be made clear that one study [55] reported measuring PIP not PE. This study defined PIP as “Prescribing that introduces a significant risk of an adverse drug-related event where there is evidence for an equally or more effective but lower-risk alternative therapy available for the same condition. Additionally, PIP includes the use of drug combinations with known drug–drug interactions, drug–disease interactions, over- dosing, use of drugs for longer time than clinically indicated, as well as lack of prescribing drugs that are clinically indicated” [31, 70]. Although both studies from where the definition was adopted described PIP in elderly people, this study included adults in their population (age 18–83 years). Additionally, PIP was categorised according to the type of decision error of the PE stage that was adapted from an ME study [71]. For these reasons, this study was classified under the category of PEs. Identified PRFs of MEs are listed in Table 5e.
All PE studies [52,53,54], except the one described earlier that reported using PIP, have consistently used the PE definition proposed by Dean et al. [72] with two of these studies [53, 54] using a modified version that encompasses mental health-specific situations. Additionally, all these studies used chart reviews to identify PEs and were prospective in nature. Data collection was performed by pharmacists [52], clinical pharmacists [54] clinical pharmacists and pharmacy technicians [53], and clinical pharmacologists [55]. Two studies reported using a standardised guidebook and training of data collectors by a pharmacy co-ordinator who facilitated data collection [53, 54]. All PE studies used multivariable logistic regression to identify PRFs of PEs except one that used Chi-square testing [52].
Several factors were investigated for their association with PEs. Two studies found that junior prescribers were less likely to make PEs compared with middle-grade and senior prescribers [53, 54]. Additionally, the same two studies found that the use of an electronic discharge pro forma increased the risk of PEs compared with handwritten prescriptions (AOR = 1.30, AOR = 1.92) [53, 54]. Non-psychotropic medications [52], increased number of medications [53, 55] and having a somatic diagnosis [55] were also reported to significantly increase the risk of PEs. As for protective factors, PEs were less likely to occur with rewritten or discharge items compared with other prescribing stages [54]. However, when PEs occurred with discharge or rewritten items, they were more likely to be clinically relevant [54].
The two studies examining MAEs [20, 21] originated from the UK and reported using the definition proposed by Barker et al. [73]. One [20], however, stated that the definition used was adapted from two additional studies [74]. Notably, both studies used direct observations to identify PRFs of MAEs. In one study [20], data were collected by pharmacists while in the other study [21], it was collected by trained pharmacists and pharmacy technicians. One used a Chi-square test and included patient-related, medication-related and hospital-related PRFs [20], while the other used Poisson regression and assessed selected hospital-related PRFs [21].
Medications that were found to be associated with a higher risk of MAEs were non-psychotropic medications and non-oral medications in one study [20]. Patient-related PRFs included having organic brain disease, incapability of consenting to medication administration, refusal to take or spitting out medications, and swallowing difficulty [20]. The latter remained significant even after the doses of crushed tablets or opened capsules were excluded (p = 0.0001).
Hospital-related PRFs included interruption of the nurse during the medication round and having to stop administering the medication to attend another ward activity [21]. This situation was found to increase the risk of MAEs by 48%. Additionally, with every increase of one ‘when required’ dose per round, the risk of error occurring in that round increased by 15% [21]. The number of patients on the ward during the round was also associated with a 6% increase in the risk of MAEs [21]. Moreover, with every one increment increase in the regular doses to be administered during the round, the rate of MAEs increased by 2% [21]. Last, errors were less likely to occur at the 22.00 hours round compared with all other rounds [20].
The only study that included more than one type of ME [51] did not define them. The data were collected by two pharmacy students supervised by two pharmacists and was analysed using the Chi-square test [51]. This study evaluated prescription errors, dose calculation errors by pharmacists, missing drugs at the time of administration, and errors of drug preparation by nurses. However, only one PRF was reported, which is the nurse preparation, as when the nurse prepared the medication immediately before administration in the care setting, MEs were more likely to occur compared with preparing medications at night for the following day. [51].
3.6 Potential Risk Factors of Medication Discrepancies
One study explored PRFs of MDs at admission [58]. Medication discrepancies at admission were defined as discrepancies on medication reconciliation, which was defined as per the American Institute for Healthcare Improvement [75]. This study identified MDs via pharmacy technician chart reviews and PRFs through a multivariable logistic regression [58]. Increasing age and number of medications were positively correlated with an increased risk of MDs. However, the gap in days between admission and medication reconciliation showed a statistically significant negative relationship indicating that an increase in the gap decreases the risk of MDs [58].
The other study investigated MDs at discharge [57]. Medication discrepancies at discharge were defined as “… any difference between the medication discharge plan and the medication administration record without supporting documentation or obvious clinical rationale” [57]. For this study, data collectors were trained, and discrepancies were identified by a panel formed of a Board-certified psychiatric pharmacist and five pharmacy students who were completing senior rotations. Medication discrepancies were identified through chart reviews and PRFs of MDs were assessed by a linear regression, which failed to identify any statistically significant PRF [57]. A complete list of tested PRFs of MDs is shown in Table 5c.
3.7 Potential Risk Factors of Drug-Related Problems
Two studies evaluated PRFs of a wider range of DRPs [59, 60]. Both studies defined DRPs as per the Pharmaceutical Care Network Europe classification [1] and used chart reviews to identify DRPs. One study indicated that data were collected by clinical pharmacists who had around 5 years’ experience with medication reviews in somatic but not mental health patients [60]. Chi-square testing was used by one study [59] to identify PRFs of DRPs while the other used the Pearson correlation [60]. The former identified increased age [59], while the latter reported the increased number of medications as PRFs of DRPs [60] (Table 5d). A complete list of all identified PRFs of all types of DRPs with statistics results can be found in the ESM.
4 Discussion
To our knowledge, this is the first systematic review to explore PRFs of DRPs in mental health inpatient units. Our findings revealed a variety of patient-related, medication-related, and hospital-related PRFs of DRPs. Patient age, sex and the number of prescribed medications were the most commonly explored PRFs in this review, which is similar to findings of a review of PRFs of serious adverse reactions in general hospitals and nursing homes [14]. The factor most consistently reported to increase the risk of most types of DRPs was an increased number of prescribed medications, echoing the findings of two reviews outside mental healthcare [14, 76]. Potentially inappropriate prescribing was the most investigated type of DRP and was consistently associated with an increased number of medications, increased duration of hospitalisation and a prior fall within the last 3 months. Consistency in PRFs of DRPs was apparent within the types and subtypes of DRPs but not across different types. This could be because of a lack of evidence or variation in the factors and circumstances involved with each type or subtype of DRP.
The present review identified some PRFs of ADRs in mental health to be analogous to acute care, including length of hospitalisation, some cardiovascular medications and some neurological medications [15, 77]. However, some PRFs differed such as increased age and female sex, which were reported as PRFs of ADRs in acute care but were associated with an inconsistent effect in this review [15, 77]. This uncertainty in the relationship between sex as well as age with DRPs was previously observed by Saedder et al. in their review of DRPs in general hospitals and nursing homes [14]. Saedder et al. argued that female patients and elderly patients may have a higher risk of DRPs primarily owing to comorbidities and an increased number of prescribed medications; implying that neither age nor sex is a risk factor per se [14]. Indeed, the studies that found increased age or female sex to be associated with a higher risk of DRPs in our review used chi-square testing, which does not account for confounders such as polypharmacy or the presence of comorbidities. Another explanation could be the variation within individual DRPs as specific items of STOPP criteria were previously found to have different associations with age in primary care [78]. Future research could focus on investigating patient age and sex within DRP types and subtypes while accounting for comorbidities and the number of prescribed medications.
Some mental health-specific PRFs were identified in this review such as the use of non-psychotropic medications. Medication errors were found to occur more frequently with non-psychotropic medications in two studies in this review (PE = 1, MAE = 1), which is similar to findings of Maidment et al. [12] in their review of MEs in elderly psychiatric patients. Maidment et al. suggested that this might be explained by mental health professionals having less familiarity with non-psychotropic medications. In contrast, Alshehri et al. [3] reported that MEs occurred more frequently with psychotropic medications in mental health, though their finding was based on counting of data rather than based on prevalence rates. One study in this review offers a possible explanation to this inconsistency. It was found that senior prescribers were likely to make more non-psychotropic errors compared with junior prescribers [53], indicating that a higher proportion of non-psychotropic errors might be related to a higher proportion of senior prescribing in the unit. Future studies may further explore this association to confirm this finding.
Studies included in this review had some shortcomings such as not including any laboratory PRFs in their analysis. Impaired renal function [14, 15, 77], liver disease [77] and increased white blood cells [77] are PRFs of DRPs in general hospitals and may be potential PRFs in psychiatry patients. Whilst patients in mental health wards may not be acutely physically unwell, older patients are at a higher risk of reduced renal function that could be accelerated with the use of some psychotropic medications such as lithium [79]. Additionally, uncontrolled blood pressure was reported as a PRF of DRPs in general hospitals [16], which may be similar in patients with mental illness who have been reported to have an increased risk of hypertension [80, 81]. The quality of the studies was also questionable as less than 10% reported all necessary research sections described in the quality assessment criteria used. The validity and reliability of methods used to identify DRPs were also questionable as less than one third of the studies had more than one healthcare professional evaluate DRPs based on a validated criterion [4, 17, 50, 52,53,54]. This is important as ADRs might be confused with the signs and symptoms of disease [76], and when ADRs were evaluated by three clinical pharmacologists in one study, they disagreed on 50% of the cases [82]. Around one quarter of the studies used the Chi-square test, which does not account for confounding factors, to test the association between the PRFs and DRPs. The importance of accounting for confounders is highlighted by the fact that in some included studies, several PRFs identified through a univariable regression did not remain significant when a multivariable regression was conducted. Furthermore, a common phenomenon was that some studies reported results that were statistically significant without reporting all tested factors. This introduces the risk of selective reporting of significant results and may prompt bias [83]. Although negative findings might be less appealing, they are powerful and add to overall knowledge of phenomena [84]. It is therefore recommended that future studies use and report robust validity and reliability measures and statistical tests, and report detailed methodology and complete findings.
Marked variability was seen between studies in terms of methodology, point of care, location, DRP types and PRFs evaluated. Such heterogeneity is not unexpected as several systematic reviews of DRPs in various settings reported similar methodological variations [77, 85,86,87,88], which preclude direct comparisons and pooling of the data. Yet, it is worth noting that some similarities exist indicating an awareness of this issue and attempts for standardisation. For example, both the MAE studies included used direct observation, which might be considered the gold standard for MAE measurement [89], whereas most studies examining other subtypes of MEs or types of DRPs used chart reviews. Moreover, almost all PE studies adopted the same definition of PEs. The marked variability observed between studies, however, underscores the need for more standardisation for PRF studies to yield homogenous results and allow for direct comparisons. Findings of this review could inform the development of a structured guide for PRF studies aiming to homogenise the methodology, type of explored factors, type of DRPs, as well as populations included.
This new knowledge of PRFs contributes to achieving patient safety goals set by WHO and the National Health Service. A key action area proposed by WHO in the third Global Patient Safety Challenge was high-risk situations and a main domain in the strategic framework was systems and practices of medications. Some PRFs identified in this review such as the number of prescribed medications could serve as indicators for high-risk situations to measure performance in benchmarking and dashboards. Additionally, these PRFs could inform strategies and approaches to tackle the systems and practices of the medication challenge domain. Several strategies have been previously proposed to reduce the risk of DRPs such as medication reconciliation and reviews, ward-based clinical pharmacists, prescriber education, avoiding the use of inappropriate medications, avoiding PRFs of ADRs, computer-based prescribing systems and computerisation of the medication process [7, 90, 91]. Risk factor data have previously been used to guide the provision of care by developing predictive scores that identify hospitalised patients at a high risk of DRPs in acute hospital settings [92,93,94]. Such predictive tools were reported to be of great value for pharmacists as it allows them to have a greater oversight of ward needs enabling them to manage workload more efficiently [95]. The results of this review could be used to develop pharmaceutical prioritisation tools to identify inpatients with mental illness in most need of medication reviews. Such an approach is much needed to optimise pharmacy services as per the Lord Carter report [96] and mitigate the risks of DRPs, ensuring more patients are in good mental and physical health. In the UK, this approach may also help in achieving the objectives of the UK mental health strategy ‘No Health Without Mental Health’ [97].
Despite rigorous adherence to systematic review guidelines such as the Cochrane Handbook for Systematic Reviews of Interventions [98], PRISMA-P [24] and the Centre for Reviews and Dissemination [42], this study has limitations. First, by not searching the grey literature, relevant reports may have been missed. However, the choice of databases was based on an evidence of search optimality [99] and the search strategy was developed in accordance with PRESS guidelines [34] and was reviewed by a librarian from the University of Manchester. Another limitation is that the screening process was led by one researcher only. Nonetheless, uncertainties during screening were resolved through a discussion with all the authors and data were independently extracted by two of the study authors. Last, although some authors were contacted for clarifications, none responded, which may have affected the clarity of reported data. A main strength in this study is that strict inclusion and exclusion criteria were used so that only studies that demonstrated PRFs of DRPs using formal statistical tests based on prevalence were included. Additionally, this review included studies published in any language to reduce the risk of language bias.
5 Conclusions
This review has synthesised current knowledge about PRFs of DRPs in mental health acute care at an international level. Patient age, sex and the number of prescribed medications were the most commonly evaluated factors for DRPs in mental health-based units. An increased number of prescribed medications was the most consistently reported factor to be significantly associated with a higher risk of most types of DRPs. Other factors were consistent within but not across individual types of DRPs. Identified PRFs could be used in conjunction with current prioritisation approaches to develop tools to identify mental health inpatients in most need of pharmaceutical care. The results indicate a lack of comprehensive evidence on PRFs of DRP in acute mental healthcare and future research should focus on determining with greater certainty the range and nature of PRFs associated with DRPs in this setting using standardised approaches.
References
Pharmaceutical Care Network Europe Foundation. The PCNE classification V 6.2. 2010. http://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf. Accessed 19 Oct 2022.
Marcus SC, Hermann RC, Frankel MR, Cullen SW. Safety of psychiatric inpatients at the Veterans Health Administration. Psych Serv. 2018;69(2):204–10. https://doi.org/10.1176/appi.ps.201700224.
Alshehri GH, Keers RN, Ashcroft DM. Frequency and nature of medication errors and adverse drug events in mental health hospitals: a systematic review. Drug Saf. 2017;40(10):871–86. https://doi.org/10.1007/s40264-017-0557-7.
Alshehri GH, Ashcroft DM, Nguyen J, Hann M, Jones R, Seaton K, et al. Prevalence, nature, severity and preventability of adverse drug events in mental health settings: findings from the MedicAtion relateD harm in mEntal health hospitals (MADE) Study. Drug Saf. 2021;44(8):877–88. https://doi.org/10.1007/s40264-021-01088-6.
van den Bemt PMLA, Egberts TCG, de Jong-van den Berg LTW, Brouwers JRBJ. Drug-related problems in hospitalised patients. Drug Saf. 2000;22(4):321–33. https://doi.org/10.2165/00002018-200022040-00005.
World Health Organisation. Medication without harm: global patient safety challenge on medication safety. Geneva: WHO; 2017. https://www.who.int/initiatives/medication-without-harm. Accessed 19 Oct 2022.
Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407. https://doi.org/10.2165/00002018-200730050-00003.
Schooling CM, Jones HE. Clarifying questions about “risk factors”: predictors versus explanation. Emerg Themes Epidemiol. 2018;15(1):10. https://doi.org/10.1186/s12982-018-0080-z.
PostScript Acute NHS Greater Glasgow and Clyde. Pharmacy prioritisation and referral. 2014. https://ggcmedicines.org.uk/media/uploads/postscript_acute/ps_acute_issue_17_june_2014.pdf. Accessed 19 Oct 2022.
Grasso BC, Rothschild JM, Genest R, Bates DW. What do we know about medication errors in inpatient psychiatry? J Commun J Qual Saf. 2003;29(8):391–400. https://doi.org/10.1016/S1549-3741(03)29047-X.
Maidment ID, Lelliott P, Paton C. Medication errors in mental healthcare: a systematic review. Qual Saf Health Care. 2006;15(6):409–13. https://doi.org/10.1136/qshc.2006.018267.
Maidment ID, Haw C, Stubbs J, Fox C, Katona C, Franklin BD. Medication errors in older people with mental health problems: a review. Int J Geriatr Psychiatry. 2008;23(6):564–73. https://doi.org/10.1002/gps.1943.
Procyshyn RM, Barr AM, Brickell T, Honer WG. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595–609. https://doi.org/10.2165/11533710-000000000-00000.
Saedder EA, Lisby M, Nielsen LP, Bonnerup DK, Brock B. Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review. Br J Clin Pharmacol. 2015;80(4):808–17. https://doi.org/10.1111/bcp.12600.
Mudigubba MK, Murthy MK, Swaroop AM, M Nayantara, Dahiya S. A systematic review of risk factors of adverse drug reactions in hospitalized patients. Asian J Pharm Clin Res. 2018;11:25. https://doi.org/10.22159/ajpcr.2018.v11i10.2775.9.
Adem F, Abdela J, Edessa D, Hagos B, Nigussie A, Mohammed MA. Drug-related problems and associated factors in Ethiopia: a systematic review and meta-analysis. J Pharm Policy Pract. 2021;14(1):36. https://doi.org/10.1186/s40545-021-00312-z.
Kanagaratnam L, Mahmoudi R, Novella JL, Jolly D, Dramé M, Trenque T. Adverse drug reactions in elderly subjects hospitalized in a specialized dementia management unit. Drugs Aging. 2014;31(10):769–76. https://doi.org/10.1007/s40266-014-0206-0.
Gummadi T, Harave VS, Aiyar LN, RajaLekshmi SG, Kunnavil R. Adverse drug reaction monitoring in a tertiary care psychiatry setting: a comparative study between inpatients and outpatients. Indian J Psychol Med. 2017;39(3):306–11. https://doi.org/10.4103/0253-7176.207328.
Mann K, Rothschild JM, Keohane CA, Chu JA, Bates DW. Adverse drug events and medication errors in psychiatry: methodological issues regarding identification and classification. World J Biol Psychiatry. 2008;9(1):24–33. https://doi.org/10.1080/15622970601178056.
Haw C, Stubbs J, Dickens G. An observational study of medication administration errors in old-age psychiatric inpatients. Int J Qual Health Care. 2007;19(4):210–6. https://doi.org/10.1093/intqhc/mzm019.
Cottney A, Innes J. Medication-administration errors in an urban mental health hospital: a direct observation study. Int J Ment Health Nurs. 2015;24(1):65–74. https://doi.org/10.1111/inm.12096.
Lucca J, Ramesh M, Ram D. Gender differences in the occurrences and pattern of adverse drug reactions in psychiatric patients: a prospective observational study. Trop J Med Res. 2017;20(1):84–90. https://doi.org/10.4103/1119-0388.198134.
Ayani N, Sakuma M, Morimoto T, Kikuchi T, Watanabe K, Narumoto J, et al. The epidemiology of adverse drug events and medication errors among psychiatric inpatients in Japan: the JADE study. BMC Psychiatry. 2016;16(1):303. https://doi.org/10.1186/s12888-016-1009-0.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349: g7647. https://doi.org/10.1136/bmj.g7647.
Offord DR, Kraemer HC. Risk factors and prevention. Evid Based Mental Health. 2000;3(3):70. https://doi.org/10.1136/ebmh.3.3.70.
Bates D, Leape L, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8:289–94. https://doi.org/10.1007/BF02600138.
World Health Organization. Safety of medicines: a guide to detecting and reporting adverse drug reactions. Geneva: World Health Organization; 2002. https://apo.who.int/publications/i/item/WHO-EDM-QSM-2002-2. Accessed 19 Oct 2022.
Suggested definitions and relationships among medication misadventures, medication errors, adverse drug events, and adverse drug reactions. Am J Health Syst Pharm. 1998;55(2):165–6. https://doi.org/10.1093/ajhp/55.2.165.
AHRQ. Medication Discrepancies and Potential Adverse Drug Events During Transfer of Care from Hospital to Home. Rockville, MD; 2017. https://www.ahrq.gov/patient-safety/resources/liability/neumiller.html. Accessed 19 Oct 2022.
Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49(2):200–9. https://doi.org/10.1046/j.1532-5415.2001.49042.x.
O’Connor MN, Gallagher P, O’Mahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging. 2012;29(6):437–52. https://doi.org/10.2165/11632610-000000000-00000.
Donaldson LJ. An organisation with a memory: report of an expert group on learning from adverse events in the NHS. London: Stationery Office; 2000.
Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, D.C: National Academy Press; 2000.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064. https://doi.org/10.1136/bmj.38636.593461.68.
Team TE. EndNote. 20th ed. Philadelphia: Clarivate; 2013.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evid Based Med. 2021;26(3):88. https://doi.org/10.1136/bmjebm-2020-111651.
Büchter RB, Weise A, Pieper D. Development, testing and use of data extraction forms in systematic reviews: a review of methodological guidance. BMC Med Res Methodol. 2020;20(1):259. https://doi.org/10.1186/s12874-020-01143-3.
Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697–703. https://doi.org/10.1016/j.jclinepi.2005.11.010.
Allan EL, Barker KN. Fundamentals of medication error research. Am J Hosp Pharm. 1990;47(3):555–71.
Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: CRD, University of York; 2009. Report No.: 9781900640473. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 19 Oct 2022.
Lang PO, Hasso Y, Dramé M, Vogt-Ferrier N, Prudent M, Gold G, et al. Potentially inappropriate prescribing including under-use amongst older patients with cognitive or psychiatric co-morbidities. Age Ageing. 2010;39(3):373–81. https://doi.org/10.1093/ageing/afq031.
Lang PO, Vogt-Ferrier N, Hasso Y, Le Saint L, Dramé M, Zekry D, et al. Interdisciplinary geriatric and psychiatric care reduces potentially inappropriate prescribing in the hospital: interventional study in 150 acutely ill elderly patients with mental and somatic comorbid conditions. J Am Med Dir Assoc. 2012;13(4):406.e1-7. https://doi.org/10.1016/j.jamda.2011.03.008.
Rongen S, Kramers C, O’Mahony D, Feuth TB, Olde Rikkert MG, Ahmed AI. Potentially inappropriate prescribing in older patients admitted to psychiatric hospital. Int J Geriatr Psychiatry. 2016;31(2):137–45. https://doi.org/10.1002/gps.4302.
Fond G, Fajula C, Dassa D, Brunel L, Lançon C, Boyer L. Potentially inappropriate psychotropic prescription at discharge is associated with lower functioning in the elderly psychiatric inpatients: a cross-sectional study. Psychopharmacology. 2016;233(13):2549–58. https://doi.org/10.1007/s00213-016-4312-z.
Wolff J, Reißner P, Hefner G, Normann C, Kaier K, Binder H, et al. Pharmacotherapy, drug-drug interactions and potentially inappropriate medication in depressive disorders. PLoS ONE. 2021;16(7): e0255192. https://doi.org/10.1371/journal.pone.0255192.
Wolff J, Hefner G, Normann C, Kaier K, Binder H, Hiemke C, et al. Polypharmacy and the risk of drug–drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiol Drug Saf. 2021;30(9):1258–68. https://doi.org/10.1002/pds.5310.
Hefner G, Hahn M, Toto S, Hiemke C, Roll SC, Wolff J, et al. Potentially inappropriate medication in older psychiatric patients. Eur J Clin Pharmacol. 2021;77(3):331–9. https://doi.org/10.1007/s00228-020-03012-w.
Seifert J, Fay B, Strueven NT, Schiekofer S, Wenzel-Seifert K, Haen E. Adverse drug reactions in geriatric psychiatric patients: influence of potentially inappropriate drugs. Psychiatr Prax. 2022;49(1):37–45. https://doi.org/10.1055/a-1394-2412.
Belkacem K, Lepaux DJ, Oliger R. Medication error rate in the hospital setting: a pilot study at the Jury-lès-Metz Hospital Center. Presse Med 1983). 2001;30(16):785–9.
Haw C, Stubbs J. Prescribing errors at a psychiatric hospital. Pharm Pract. 2003;13(2):64.
Keers RN, Williams SD, Vattakatuchery JJ, Brown P, Miller J, Prescott L, et al. Medication safety at the interface: evaluating risks associated with discharge prescriptions from mental health hospitals. J Clin Pharm Ther. 2015;40(6):645–54. https://doi.org/10.1111/jcpt.12328.
Keers RN, Williams SD, Vattakatuchery JJ, Brown P, Miller J, Prescott L, et al. Prevalence, nature and predictors of prescribing errors in mental health hospitals: a prospective multicentre study. BMJ Open. 2014;4(9):e006084-e. https://doi.org/10.1136/bmjopen-2014-006084.
Soerensen AL, Nielsen LP, Poulsen BK, Lisby M, Mainz J. Potentially inappropriate prescriptions in patients admitted to a psychiatric hospital. Nord J Psychiatry. 2016;70(5):365–73. https://doi.org/10.3109/08039488.2015.1127996.
Dharman D, Krishnan SP, Ravikumar K. Prevalence, pattern and monitoring of adverse drug reaction in tertiary care psychiatry setting: a hospital based study in South Kerala. J Pharm Res. 2021;2021:35–46.
Nelson LA, Graham MR, Schaefer MG. Characterization of medication discrepancies occurring at the time of discharge from an adult state psychiatric inpatient facility. Hosp Pharm. 2011;46(4):254–61. https://doi.org/10.1310/hpj4604-254.
Brownlie K, Schneider C, Culliford R, Fox C, Boukouvalas A, Willan C, et al. Medication reconciliation by a pharmacy technician in a mental health assessment unit. Int J Clin Pharm. 2014;36(2):303–9. https://doi.org/10.1007/s11096-013-9875-8.
Mateti UV, Lalwani T, Nagappa AN, Bhandary PV, Verupaksha D, Balkrishnan R. Assessment of drug-related problems in depressive patients. Perspect Clin Res. 2015;6(1):58–61. https://doi.org/10.4103/2229-3485.148820.
Kibsdal KP, Andersen S, Gazerani P, Plet H. Rates and correlates of pharmacotherapy-related problems among psychiatric inpatients: a representative Danish study. Ther Adv Psychopharmacol. 2020;10:2045125320957120. https://doi.org/10.1177/2045125320957120.
Holt S, Schmiedl S, Thürmann PA. Potenziell inadäquate medikation für ältere menschen: die PRISCUS-liste. Deutsches Ärzteblatt (Ausg A). 2010;107(31–32):543–51. https://doi.org/10.3238/arztebl.2010.0543.
Lang P-O, Hasso Y, Belmin J, Payot I, Baeyens J-P, Vogt-Ferrier N, et al. STOPP-START: Adaptation en langue française d’un outil de détection de la prescription médicamenteuse inappropriée chez la personne âgée. Can J Public Health. 2009;100(6):426–31. https://doi.org/10.1007/BF03404338.
Fialová D, Topinková E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, et al. Potentially inapproriate medication use among elderly home care patients in Europe. JAMA. 2005;293(11):1348–58. https://doi.org/10.1001/jama.293.11.1348.
Laroche M-L, Charmes J-P, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–31. https://doi.org/10.1007/s00228-007-0324-2.
Campanelli CM. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults: the American Geriatrics Society 2012 Beers Criteria Update Expert Panel. J Am Geriatr Soc. 2012;60(4):616–31. https://doi.org/10.1111/j.1532-5415.2012.03923.x.
Am VW-vdT, Mm V, Hj D, Rj vM. [Detection of inappropriate medication use in the elderly; will the STOPP and START criteria become the new Dutch standards?]. Ned Tijdschr Geneeskd. 2012;156(40):A5076.
Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801. https://doi.org/10.7326/0003-4819-140-10-200405180-00009.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29–34. https://doi.org/10.1001/jama.1995.03530010043033.
Institute for Healthcare Improvement. Trigger tool for measuring adverse drug events in a mental health setting. 2008. http://www.ihi.org/resources/Pages/Tools/TriggerToolMeasuringADEsinMentalHealthSetting.aspx. Accessed 19 Oct 2022.
Gallagher P, Barry P, O’Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther. 2007;32(2):113–21. https://doi.org/10.1111/j.1365-2710.2007.00793.x.
Lisby M, Nielsen LP, Brock B, Mainz J. How should medication errors be defined? Development and test of a definition. Scand J Public Health. 2012;40(2):203–10. https://doi.org/10.1177/1403494811435489.
Dean B, Barber N, Schachter M. What is a prescribing error? Qual Health Care. 2000;9(4):232–7. https://doi.org/10.1136/qhc.9.4.232.
Barker KN, Flynn EA, Pepper GA, Bates DW, Mikeal RL. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897–903. https://doi.org/10.1001/archinte.162.16.1897.
O’Shea E. Factors contributing to medication errors: a literature review. J Clin Nurs. 1999;8(5):496–504. https://doi.org/10.1046/j.1365-2702.1999.00284.x.
Institute for Healthcare Improvement. Accuracy at every step: the challenge of medication reconciliation. Cambridge, MA: Institute of Healthcare Improvement; 2006. http://www.ihi.org/resources/pages/improvementstories/accuracyateverystep.aspx. Accessed 19 Oct 2022.
Ayele Y, Tesfaye ZT. Drug-related problems in Ethiopian public healthcare settings: systematic review and meta-analysis. SAGE Open Med. 2021;9:20503121211009730. https://doi.org/10.1177/20503121211009728.
Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86. https://doi.org/10.2147/cia.S71178.
Bradley MC, Fahey T, Cahir C, Bennett K, O’Reilly D, Parsons C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur J Clin Pharmacol. 2012;68(10):1425–33. https://doi.org/10.1007/s00228-012-1249-y.
Rej S, Li BW, Looper K, Segal M. Renal function in geriatric psychiatry patients compared to non-psychiatric older adults: effects of lithium use and other factors. Aging Mental Health. 2014;18(7):847–53. https://doi.org/10.1080/13607863.2014.888536.
Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45–53. https://doi.org/10.1016/j.schres.2005.08.010.
Aguglia A, Salvi V, Amerio A, Gari M, Dragogna F, Mencacci C, et al. Number of episodes and duration of illness associated with hypertension and 10-year cardiovascular risk in patients with bipolar disorder type I. Psychiatry Res. 2022;308: 114344. https://doi.org/10.1016/j.psychres.2021.114344.
Karch FE, Smith CL, Kerzner B, Mazzullo JM, Weintraub M, Lasagna L. Adverse drug reactions: a matter of opinion. Clin Pharmacol Ther. 1976;19(5part1):489–92. https://doi.org/10.1002/cpt1976195part1489.
Hutton JL, Williamson PR. Bias in meta-analysis due to outcome variable selection within studies. J R Stat Soc Se C Appl Stat. 2000;49(3):359–70. https://doi.org/10.1111/1467-9876.00197.
Hilten LGV. Why it’s time to publish research “failures”. Publishing bias favors positive results; now there’s a movement to change that. Amsterdam: Elsevier Connect; 2015.
Keers RN, Williams SD, Cooke J, Walsh T, Ashcroft DM. Impact of interventions designed to reduce medication administration errors in hospitals: a systematic review. Drug Saf. 2014;37(5):317–32. https://doi.org/10.1007/s40264-014-0152-0.
Alghamdi AA, Keers RN, Sutherland A, Ashcroft DM. Prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care settings: a systematic review. Drug Saf. 2019;42(12):1423–36. https://doi.org/10.1007/s40264-019-00856-9.
Keers RN, Williams SD, Cooke J, Ashcroft DM. Causes of medication administration errors in hospitals: a systematic review of quantitative and qualitative evidence. Drug Saf. 2013;36(11):1045–67. https://doi.org/10.1007/s40264-013-0090-2.
Alsulami Z, Conroy S, Choonara I. Medication errors in the Middle East countries: a systematic review of the literature. Eur J Clin Pharmacol. 2013;69(4):995–1008. https://doi.org/10.1007/s00228-012-1435-y.
Flynn EA, Barker KN, Pepper GA, Bates DW, Mikeal RL. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am J Health Syst Pharm. 2002;59(5):436–46. https://doi.org/10.1093/ajhp/59.5.436.
Onder G, van der Cammen TJM, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42(3):284–91. https://doi.org/10.1093/ageing/aft038.
Khalil H, Kynoch K, Hines S. Interventions to ensure medication safety in acute care: an umbrella review. JBI Evidence Implementation. 2020;18(2)
Urbina O, Ferrández O, Grau S, Luque S, Mojal S, Marin-Casino M, et al. Design of a score to identify hospitalized patients at risk of drug-related problems. Pharmacoepidemiol Drug Saf. 2014;23(9):923–32. https://doi.org/10.1002/pds.3634.
Alshakrah MA, Steinke DT, Tully MP, Abuzour AS, Williams SD, Lewis PJ. Development of the adult complexity tool for pharmaceutical care (ACTPC) in hospital: a modified Delphi study. Res Soc Admin Pharm. 2021. https://doi.org/10.1016/j.sapharm.2021.02.009.
Nazanin F, Sanjoy N, Doreen L, Aaron J, Mary S. Development of an electronic patient prioritization tool for clinical pharmacist interventions. Am J Health Syst Pharm. 2014;71(4):311–20. https://doi.org/10.2146/ajhp130247.
Abuzour AS, Hoad-Reddick G, Shahid M, Steinke DT, Tully MP, Williams SD, et al. Patient prioritisation for hospital pharmacy services: current approaches in the UK. Eur J Hosp Pharm. 2020. https://doi.org/10.1136/ejhpharm-2020-002365.
Carter PR. Lord Carter’s review into unwarranted variations in mental health and community health services. National Health Services; 2018. https://www.england.nhs.uk/publication/lord-carters-review-into-unwarranted-variations-in-mental-health-and-community-health-services/
Department of Health. No health, without mental health: a cross-government mental health outcomes strategy for people of all ages. 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/138253/dh_124058.pdf. Accessed 19 Oct 2022.
Higgins JPT Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane; 2021. www.training.cochrane.org/handbook. Accessed 19 Oct 2022.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):245. https://doi.org/10.1186/s13643-017-0644-y.
Acknowledgements
The authors acknowledge Sanaa Idir for translating the French study and Katharina Wien for translating the German study. The authors also thank Michael Stevenson (University of Manchester librarian) for reviewing the search strategy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Conflicts of interest/competing interests
Fatima Q. Alshaikhmubarak, Richard N. Keers and Penny J. Lewis have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
This systematic review was part of FQA’s PhD project supervised by PJL and RNK. All the authors contributed to the conceptualisation of the research idea and development of the protocol. The search and screening of the articles, data extraction, analysis, and writing of the manuscript were led and performed by FQA supported by PJL and RNK. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alshaikhmubarak, F.Q., Keers, R.N. & Lewis, P.J. Potential Risk Factors of Drug-Related Problems in Hospital-Based Mental Health Units: A Systematic Review. Drug Saf 46, 19–37 (2023). https://doi.org/10.1007/s40264-022-01249-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01249-1