Abstract
Background and Objectives
Selatogrel is a potent, reversible, and selective antagonist of the platelet P2Y12 receptor currently developed for the treatment of acute myocardial infarction (AMI). In the completed Phase I/II studies, selatogrel was subcutaneously (s.c.) administered as a lyophilizate-based formulation by syringe by a healthcare professional. In the Phase III study, selatogrel will be self-administered s.c. as a liquid formulation with an autoinjector at the onset of AMI symptoms to shorten treatment delay. This clinical bridging study compared the pharmacokinetics (PK) of selatogrel between the different formulations.
Methods
This was a single-center, randomized, open-label, three-period, cross-over Phase I study in 24 healthy subjects. In each period, a single subcutaneous dose of 16 mg selatogrel was administered as (1) a Phase III liquid formulation by autoinjector (Treatment A), (2) a Phase III liquid formulation by prefilled syringe (Treatment B), or (3) a Phase I/II reconstituted lyophilizate-based formulation by syringe (Treatment C). PK parameters including area under the plasma concentration–time curve from zero to infinity (AUC0–∞), maximum plasma concentration (Cmax), time to reach Cmax(tmax), and terminal half-life (t1/2) were determined using noncompartmental analysis. Pharmacodynamic (PD) parameters were estimated using PK/PD modeling, including the time of first occurrence of inhibition of platelet aggregation (IPA) ≥ 80% (tonset), duration of IPA above 80% (tduration), and responder rate defined as the percentage of subjects with tonset ≤ 30 min and tduration ≥ 3 h. Safety and tolerability were also assessed.
Results
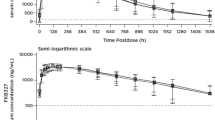
Comparing Treatment A to Treatment C, the exposure (AUC0–∞) was bioequivalent with a geometric mean ratio (GMR) (90% confidence interval) of 0.95 (0.92–0.97) within the bioequivalence range (0.80–1.25). Absorption following Treatment A was slightly slower with a tmax occurring approximately 30 min later and a 20% lower Cmax. The autoinjector itself had no impact on the PK of selatogrel, as similar values of Cmax and AUC0–∞ were determined after administration as a Phase III liquid formulation by autoinjector or by prefilled syringe (i.e., GMR [90% confidence interval] of 1.06 [0.97–1.15] and 0.99 [0.96–1.03] for Cmax and AUC0–∞, respectively). PK/PD modeling predicted that the median tonset will occur slightly later for Treatment A (7.2 min) compared to Treatment C (4.2 min), while no relevant differences in tduration and responder rate were estimated between the two treatments. Selatogrel was safe and well tolerated following all three treatments.
Conclusions
PK and simulated PD effects of selatogrel were similar across treatments.
Clinical Trial Registration
NCT04557280.


Similar content being viewed by others
References
O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485–510.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with Non-ST-Elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-228.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–77.
Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9(2):154–69.
Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42(14):1289–367.
Juif PE, Boehler M, Dobrow M, Ufer M, Dingemanse J. Clinical pharmacology of the reversible and potent P2Y12 receptor antagonist ACT-246475 after single subcutaneous administration in healthy male subjects. J Clin Pharmacol. 2019;59(1):123–30.
Sinnaeve P, Fahrni G, Schelfaut D, Spirito A, Mueller C, Frenoux JM, et al. Subcutaneous selatogrel inhibits platelet aggregation in patients with acute myocardial infarction. J Am Coll Cardiol. 2020;75(20):2588–97.
Storey RF, Gurbel PA, ten Berg J, Bernaud C, Dangas GD, Frenoux JM, et al. Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur Heart J. 2020;41(33):3132–40.
Nabel EG, Braunwald E. A Tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54–63.
Dracup K. The challenge of reducing prehospital delay in patients with acute coronary syndrome. Cir Cardiovasc Qual Outcomes. 2009;2(3):144–5.
Nilsson G, Mooe T, Söderström L, Samuelsson E. Pre-hospital delay in patients with first time myocardial infarction: an observational study in a northern Swedish population. BMC Cardiovasc Disord. 2016;16:93. https://doi.org/10.1186/s12872-016-0271-x.
Schilling U, Dingemanse J, Voors-Pette C, Romeijn C, Dogterom P, Ufer M. Effect of rifampin-mediated OATP1B1 and OATP1B3 transporter inhibition on the pharmacokinetics of the P2Y12 receptor antagonist selatogrel. Clin Transl Sci. 2020;13(5):886–90.
Schilling U, Dingemanse J, Dobrow M, Baumann M, Riederer MA, Juif PE, et al. Insights from in vitro and clinical data to guide transition from the novel P2Y12 antagonist selatogrel to clopidogrel, prasugrel, and ticagrelor. Thromb Haemost. 2021;121(6):755–66. https://doi.org/10.1055/s-0040-1721773.
Ufer M, Huynh C, van Lier JJ, Caroff E, Fischer H, Dingemanse J. Absorption, distribution, metabolism and excretion of the P2Y12 receptor antagonist selatogrel after subcutaneous administration in healthy subjects. Xenobiotica. 2020;50(4):427–34.
Baldoni D, Bruderer S, Krause S, Gutierrez M, Gueret P, Astruc B, et al. A new reversible and potent P2Y12 receptor antagonist (ACT-246475): tolerability, pharmacokinetics, and pharmacodynamics in a first-in-man trial. Clin Drug Investig. 2014;34(11):807–18.
ClinicalTrials.gov. The effect of reduced liver function on selatogrel pharmacokinetics. https://clinicaltrials.gov/ct2/show/NCT04406896. Accessed 26 Jul 2021.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49(4):1020–38.
Gaglia MA, Torguson R, Pakala R, Xue Z, Sardi G, Suddath WO, et al. Correlation between light transmission aggregometry, VerifyNow P2Y12, and VASP-P platelet reactivity assays following percutaneous coronary intervention. J Interv Cardiol. 2011;24(6):529–34.
Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate aspirin-mediated platelet inhibition after percutaneous intervention with stent implantation. Platelets. 2011;22(3):188–95.
Henrich A, Claussen CH, Dingemanse J, Krause A. Pharmacokinetic/pharmacodynamic modeling of drug interactions at the P2Y12 receptor between selatogrel and oral P2Y12 antagonists. CPT Pharmacometr Syst Pharmacol. 2021;10(7):735–47.
Schilling U, Dingemanse J, Ufer M. Pharmacokinetics and pharmacodynamics of approved and investigational P2Y12 receptor antagonists. Clin Pharmacokinet. 2020;59(5):545–66.
ClinicalTrials.gov. Selatogrel outcome study in suspected acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT04957719. Accessed 26 Jul 2021.
Acknowledgements
The authors thank Mark Enzler from Swiss BioQuant AG, Reinach, Switzerland for the bioanalytical conduct of this study and Beya Khouildi, Anne-Sophie Guern, and Anna Kaufmann for their dedicated support in the conduct of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Idorsia Pharmaceuticals Ltd.
Conflict of interest
During the conduct of this study, IZ, CH, US, AK, MU, and JD were full-time employees of Idorsia Pharmaceuticals Ltd and MK was the principal investigator.
Ethics approval
The protocol was approved by the Midlands Independent Review Board, USA. This study was performed according to Good Clinical Practice and in accordance with the principles of the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent prior to the initiation of study-related procedures.
Consent for publication
Not applicable (no personal data were reported).
Data availability
Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The model codes are available upon request.
Author contributions
IZ wrote the manuscript, CH and AK conducted the PK/PD modeling, US, MU, and JD designed and analyzed the study, and MK was the principal investigator of the study. All authors revised the manuscript critically for important intellectual content and approved the version to be submitted.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zenklusen, I., Hsin, CH., Schilling, U. et al. Transition from Syringe to Autoinjector Based on Bridging Pharmacokinetics and Pharmacodynamics of the P2Y12 Receptor Antagonist Selatogrel in Healthy Subjects. Clin Pharmacokinet 61, 687–695 (2022). https://doi.org/10.1007/s40262-021-01097-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01097-9