Abstract
Background and Objective
Approximately 38 million people worldwide experience heart failure (HF), with more than 10 million in China. Heart failure exacerbations are the main cause of HF hospitalization, and hospitalizations are the main driver of HF-associated costs. Vericiguat is recommended to treat patients who have had worsening HF despite guideline-directed medical therapy. However, the cost effectiveness of adding vericiguat to the standard treatment of this population in China remains unclear. The objective of this study was to investigate the cost effectiveness of adding vericiguat to standard treatment in patients with HF in the Chinese population
Methods
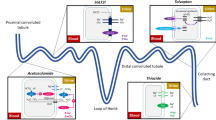
A lifetime Markov model with a 1-month cycle length was developed to compare the cost effectiveness of vericiguat plus standard treatment versus standard treatment alone in Chinese patients with HF with reduced ejection fraction following an HF exacerbation, from the perspective of Chinese healthcare providers. The clinical data were obtained from the VICTORIA study. The cost was accessed from our institution or studies conducted in China. The primary outcome was the incremental cost-effectiveness ratio, representing incremental cost per incremental quality-adjusted life-year (QALY). Vericiguat was considered highly cost effective if the incremental cost-effectiveness ratio obtained was lower than 12,551 USD/QALY, cost effective if the incremental cost-effectiveness ratio was between 12,551 and 37,654.5 USD/QALY, and not cost effective if the incremental cost-effectiveness ratio was higher than 37,654.5 USD/QALY. A scenario analysis, one-way sensitivity analysis, and probabilistic sensitivity analysis were performed to test the robustness of the results.
Results
For a 67-year-old patient with HF following an HF exacerbation, the lifetime cost was 17,721 USD if vericiguat plus standard treatment was given, compared to 7907 USD if standard treatment alone was prescribed. The corresponding effectiveness was 2.20 QALY and 2.10 QALY, respectively. The incremental cost-effectiveness ratio of vericiguat plus standard treatment versus standard treatment alone in Chinese patients with HF was 89,429 USD/QALY, higher than the willingness-to-pay threshold of 37654.5 USD/QALY. The scenario analysis and sensitivity analysis showed the robustness of our results.
Conclusions
The addition of vericiguat to the treatment regimen of Chinese patients with HF with reduced ejection fraction following an HF exacerbation resulted in an incremental cost-effectiveness ratio of $89,429 USD/QALY compared to standard treatment. This incremental cost-effectiveness ratio exceeds the willingness-to-pay threshold and thus, vericiguat was deemed not cost effective in the Chinese population.




Similar content being viewed by others

References
Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504. https://doi.org/10.1001/jama.2020.10262.
Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail. 2019;21(11):1329–37. https://doi.org/10.1002/ejhf.1629.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. https://doi.org/10.1038/nrcardio.2017.65.
Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, et al. Health-related quality of life and mortality in heart failure: the Global Congestive Heart Failure Study of 23 000 Patients from 40 countries. Circulation. 2021;143(22):2129–42. https://doi.org/10.1161/circulationaha.120.050850.
Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, et al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2017;19(12):1624–34. https://doi.org/10.1002/ejhf.945.
Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574–85. https://doi.org/10.1002/ejhf.813.
Zou CH, Wang YH, Zhai M, Huang Y, Zhou Q, Zhuang XF, et al. 2020 Clinical performance and quality measures for heart failure in China. Chin Circ J. 2021;36(03):221–38.
Wang H, Chai K, Du MH, Wang SF, Cai JP, Li YY, et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. 2021;14(10):1127–35. https://doi.org/10.1161/circheartfailure.121.008406.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-743. https://doi.org/10.1161/cir.0000000000000950.
Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12(4):220–9. https://doi.org/10.1038/nrcardio.2015.14.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):E876–94. https://doi.org/10.1161/cir.0000000000001062.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28. https://doi.org/10.1056/NEJMoa2030183.
Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539–48. https://doi.org/10.1056/NEJMoa1812851.
Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568–74. https://doi.org/10.1038/s41591-021-01659-1.
Alsumali A, Djatche LM, Briggs A, Liu R, Diakite I, Patel D, et al. Cost effectiveness of vericiguat for the treatment of chronic heart failure with reduced ejection fraction following a worsening heart failure event from a US Medicare Perspective. Pharmacoeconomics. 2021;39(11):1343–54. https://doi.org/10.1007/s40273-021-01091-w.
Lou YK, Yu Y, Liu JX, Huang J. Sacubitril-valsartan for the treatment of hypertension in China: a cost-utility analysis based on meta-analysis of randomized controlled trials. Front Public Health. 2022. https://doi.org/10.3389/fpubh.2022.959139.
Markham A, Duggan S. Vericiguat: first approval. Drugs. 2021;81(6):721–6. https://doi.org/10.1007/s40265-021-01496-z.
Drug data. https://db.yaozh.com/Search?typeid=8446&content=vericiguat. Accessed 28 Sep 2022.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40(6):601–9. https://doi.org/10.1007/s40273-021-01112-8.
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–93. https://doi.org/10.1056/NEJMoa1915928.
Liao CT, Yang CT, Toh HS, Chang WT, Chang HY, Kuo FH, et al. Cost-effectiveness evaluation of add-on dapagliflozin for heart failure with reduced ejection fraction from perspective of healthcare systems in Asia-Pacific region. Cardiovasc Diabetol. 2021;20(1):204. https://doi.org/10.1186/s12933-021-01387-3.
Liao CT, Yang CT, Kuo FH, Lee MC, Chang WT, Tang HJ, et al. Cost-effectiveness evaluation of add-on empagliflozin in patients with heart failure and a reduced ejection fraction from the healthcare system’s perspective in the Asia-Pacific region. Front Cardiovasc Med. 2021;8: 750381. https://doi.org/10.3389/fcvm.2021.750381.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190.
Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease results of the VERTIS CV Trial. Circulation. 2020;142(23):2205–15. https://doi.org/10.1161/circulationaha.120.050255.
Krittayaphong R, Permsuwan U. Cost-utility analysis of sacubitril-valsartan compared with enalapril treatment in patients with acute decompensated heart failure in Thailand. Clin Drug Investig. 2021;41(10):907–15. https://doi.org/10.1007/s40261-021-01079-6.
Ge Y, Zhang L, Gao Y, Wang B, Zheng X. Socio-economic status and 1 year mortality among patients hospitalized for heart failure in China. ESC Heart Fail. 2022;9(2):1027–37. https://doi.org/10.1002/ehf2.13762.
Ma XW, Yu XJ, Wang HD, Wang B, Mao QA, Liu JF, et al. China health statistics yearbook (2021): in Chinese. Beijing: Peking Union Medical College Press; 2022.
Hu SL, Wu JH, Wu J, Dong CH, Li HC, Liu GE. China guidelines for pharmacoeconomic evaluations: Chinese-English version. 1st ed. Beijing: China Market Press; 2020.
Xuan JW, Tao LB, Zhu SQ, Zhang ML, Ni Q, Sun Q, et al. Real world survey of non-direct medical cost and quality of life for heart failure patients of China. China Health Insur. 2017;10(03):61–4. https://doi.org/10.19546/j.issn.1674-3830.2017.3.013.
Lin X, Lin M, Liu M, Huang W, Nie X, Chen Z, et al. Cost-effectiveness of empagliflozin as a treatment for heart failure with reduced ejection fraction: an analysis from the Chinese healthcare perspective. J Thorac Dis. 2022;14(5):1588–97. https://doi.org/10.21037/jtd-22-463.
Jiang YH, Zheng RJ, Sang HQ. Cost-effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.733681.
Ma L, Wang Z, Fan J, Hu S. Report on cardiovascular health and diseases in China 2021: an updated summary. Chinese Circ J. 2022;37(06):553–78.
Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, et al. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA Trial. ESC Heart Fail. 2018;6(2):96–104. https://doi.org/10.1016/j.jchf.2017.08.013.
Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart Failure (China-HF) Registry. J Card Fail. 2017;23(12):868–75. https://doi.org/10.1016/j.cardfail.2017.09.014.
Jiang Y, Xie J. Cost-effectiveness of adding empagliflozin to the standard therapy for heart failure with preserved ejection fraction from the perspective of healthcare systems in China. Front Cardiovasc Med. 2022;9: 946399. https://doi.org/10.3389/fcvm.2022.946399.
Wu Y, Tian S, Rong PP, Zhang F, Chen Y, Guo XX, et al. Sacubitril-valsartan compared with enalapril for the treatment of heart failure: a decision-analytic Markov model simulation in China. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.01101.
Tang M, He J, Chen M, Cong L, Xu Y, Yang Y, et al. “4+7” city drug volume-based purchasing and using pilot program in China and its impact. Drug Discov Ther. 2019;13(6):365–9. https://doi.org/10.5582/ddt.2019.01093.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Science and Technology Program of Xi’an [No. 21YXYJ0095], the Key Industrial Innovation Chain Project in Shaanxi Province of China [No. 2020ZDLSF01-08], the Shaanxi Provincial Health and Health Research Fund Project [No. 2022D024], the Natural Science Foundation of Shaanxi Province [No. 2022SF-476], and the Key Basic Natural Science Foundation of Shaanxi Province [No. 2022JZ-47].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Code availability
Not applicable.
Author contributions
FL came up with the idea and designed the protocol. XY and YH synthesized the data and drafted the manuscript. YH developed the model. ZZ, WZ, BL, MM, XZ, NW, and JW participated in the data collection and data analysis. All authors approved the final version of the manuscript. XY and YH contributed equally to the manuscript and share the first authorship.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, X., Hao, Y., Zhu, Z. et al. Vericiguat for the Treatment of Heart Failure with Reduced Ejection Fraction Following a Worsening Heart Failure Event: A Cost-Effectiveness Analysis from the Perspective of Chinese Healthcare Providers. Clin Drug Investig 43, 241–250 (2023). https://doi.org/10.1007/s40261-023-01253-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01253-y