Abstract
Background and Objective
Perampanel is a once-daily oral anti-seizure medication indicated for focal-onset seizures and generalized tonic-clonic seizures. This study investigated the single- and multiple-dose pharmacokinetics of perampanel in healthy Chinese adults.
Methods
Study 052 (NCT03424564) was a phase I, single-center, open-label, parallel-group study. In the single-dose part of the study, subjects received a single oral dose of perampanel 2, 4, or 8 mg. In the multiple-dose part, subjects received once-daily oral perampanel 2 mg on Days 1–7 and 4 mg on Days 8–21. Pharmacokinetic parameters were determined from perampanel plasma concentrations using non-compartmental analysis. Dose proportionality after single doses of perampanel was assessed. Safety and tolerability were evaluated.
Results
In the single-dose part (N = 30), median time to reach maximum concentration (tmax) was 0.75–1.0 h, mean terminal elimination phase half-life (t½) was 85.6–122 h, mean maximum observed concentration (Cmax) was 77.9–276 ng/mL, and mean area under the concentration–time curve from time zero to time of the last quantifiable concentration (AUC(0-t)) was 4070–15100 ng·h/mL. Single-dose pharmacokinetics were linear for perampanel 2–8 mg. In the multiple-dose part (N = 12), Day 21 steady-state (4 mg/day) parameters were median time at which the highest drug concentration occurs at steady state (tss,max), 1.25 h; mean t½, 109 h; mean maximum observed concentration at steady state (Css,max), 453 ng/mL; and mean area under the concentration–time curve over the dosing interval on multiple dosing (AUC(0- τ)), 7540 ng·h/mL. For single- and multiple-dose perampanel, the most common treatment-emergent adverse events were dizziness and somnolence.
Conclusions
Single- and multiple-dose pharmacokinetics of perampanel in healthy Chinese adults revealed rapid perampanel absorption, slow elimination, and a linear relationship with single perampanel doses of 2–8 mg. Findings were consistent with previous studies of perampanel pharmacokinetics in other ethnic/racial populations of healthy subjects. Single and multiple doses of perampanel were generally safe and well tolerated.
Clinical Trial Registration
NCT03424564; registered February 2018.
Similar content being viewed by others
The single- and multiple-dose pharmacokinetics of oral perampanel were investigated in healthy Chinese subjects. |
Perampanel was rapidly absorbed and slowly eliminated after single and multiple dosing, and the pharmacokinetics of single-dose perampanel were linear across the dose range of 2–8 mg. |
Results were comparable with those reported previously for White, Japanese, and Korean subjects. |
1 Introduction
Perampanel, a selective, non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist [1], is a once-daily oral anti-seizure medication (ASM) for focal-onset seizures and generalized tonic-clonic seizures [2,3,4]. In the US, perampanel is approved for the treatment of focal-onset seizures (adjunctive and monotherapy), with or without focal to bilateral tonic-clonic seizures, in patients aged ≥ 4 years, and as adjunctive treatment of generalized tonic-clonic seizures in patients aged ≥ 12 years [3]; in Europe, perampanel is indicated for adjunctive treatment of focal-onset seizures, with or without focal to bilateral tonic-clonic seizures, in patients aged ≥ 4 years, and generalized tonic-clonic seizures in patients aged ≥ 7 years with idiopathic generalized epilepsy [2]; and in China, perampanel is available as monotherapy and adjunctive treatment for focal-onset seizures, with or without focal to bilateral tonic-clonic seizures, in patients with epilepsy aged ≥ 4 years [4].
The safety and efficacy of adjunctive perampanel in patients with focal-onset seizures have been demonstrated in several double-blind, randomized, placebo-controlled, phase III studies that included patients from North and South America, Europe, and Asia-Pacific (including China) [5,6,7,8]. An open-label extension study confirmed the long-term (up to 4 years) safety, tolerability, and seizure outcomes of adjunctive perampanel for the treatment of focal-onset seizures [9]. A separate phase III study confirmed the safety and efficacy of adjunctive perampanel for the treatment of generalized tonic-clonic seizures in patients from North America, Europe, and Asia-Pacific (including China) [10].
Previous studies conducted in both healthy subjects and patients with epilepsy have shown that perampanel is rapidly absorbed, with a maximum observed concentration (Cmax) occurring 1 h (median) after oral administration under fasted conditions in healthy subjects and 0.5–2.0 h in patients with epilepsy [11]. Although the elimination half-life of perampanel shows wide variation among healthy subjects in phase I studies, the mean value is estimated to be approximately 105 h based on a population pharmacokinetics analysis and nonlinear mixed effects modeling of data from phase I studies [11]. Dose linearity of perampanel pharmacokinetics over the range of 0.2–36 mg has been demonstrated [11].
Due to the potential effects of race and/or ethnicity on the pharmacokinetics or pharmacodynamics of drugs, evaluation in healthy populations representative of their target clinical population can provide essential information for prescribers and regulatory bodies [12]. The pharmacokinetics of multiple-dose oral perampanel have been investigated previously in healthy White, Japanese, and Korean male adults, and no clinically relevant differences between these populations were observed [13]. Although Chinese patients with focal-onset seizures with sparse sampling pharmacokinetic data have been included in a population-based pharmacokinetic analysis of perampanel [14], no specific study of perampanel in Chinese individuals has been reported to date. Although Chinese patients accounted for 15.0% of the study population [14], there is interest in broadening the understanding of perampanel pharmacokinetics, and as such, a study was undertaken to evaluate the single- and multiple-dose pharmacokinetics of perampanel in healthy Chinese adults with traditional pharmacokinetic analyses.
2 Methods
2.1 Study Overview
Study 052 (ClinicalTrials.gov identifier: NCT03424564) was a phase I, single-center, open-label, parallel-group study of single and multiple doses of perampanel in healthy Chinese subjects. Study protocols and informed consent forms were reviewed and approved by the Institutional Review Board of Beijing Anzhen Hospital. All participants provided written informed consent prior to participation in the study, which also included consent for publication.
The primary study objective was to evaluate the pharmacokinetics of perampanel following single and multiple oral doses of perampanel in healthy Chinese subjects. The secondary objectives were to evaluate the dose proportionality of perampanel pharmacokinetics following a single oral dose of perampanel, and the safety and tolerability of perampanel following single and multiple oral doses of perampanel in healthy Chinese subjects.
2.2 Subjects
Healthy, non-smoking male and female Chinese adults aged ≥ 18 years and ≤ 45 years with a body mass index of ≥ 18.5 and < 24.5 kg/m2 were eligible to participate in the study. Key exclusion criteria included weight < 50 kg; females of childbearing potential with no use of effective contraception within 28 days prior to study entry, or who were breastfeeding or pregnant during the Screening or Baseline Period; clinically significant illness that required medical treatment within 8 weeks or a clinically significant infection that required medical treatment within 4 weeks before first dosing; and any history of gastrointestinal surgery that may affect the pharmacokinetic profile of perampanel. The use of prescription medications within 4 weeks before first dosing and throughout the study was prohibited except for pre-specified, highly effective contraceptives. In addition, the following restrictions also applied: intake of over-the-counter medications or nutritional supplements, juice, and other foods or beverages that may affect various drug-metabolizing enzymes and transporters (including alcohol and grapefruit-containing foods or beverages) within 2 weeks before first dosing and throughout the study; intake of herbal preparation within 4 weeks before first dosing and throughout the study; use of tobacco or nicotine-containing products from screening before first dosing and throughout the study; and intake of caffeinated beverages or food within 72 h before first dosing until 72 h post-dose.
2.3 Study Design and Procedures
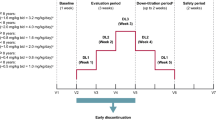
2.3.1 Single-Dose Administration
The Pretreatment Phase consisted of a Screening Period (Days − 21 to − 2) and a Baseline Period (Day − 1), and the Treatment Phase consisted of a 15-day Treatment Period (Days 1–15). Subjects were allocated to one of three open-label, parallel dose groups (2, 4, or 8 mg) in a 1:1:1 ratio and received a single oral dose of perampanel 2 mg (one 2-mg tablet), 4 mg (two 2-mg tablets), or 8 mg (four 2-mg tablets), respectively, under fasted conditions (≥ 10 h overnight) on Day 1. Subjects were confined to the study site on Days − 1 to 5, and were financially compensated for the temporary loss of working time when confined to study sites as described in the informed consent form. Afterwards, subjects had follow-up outpatient visits on Days 8, 11, and 15.
2.3.2 Multiple-Dose Administration
The Pretreatment Phase consisted of a Screening Period (Days − 21 to − 2) and a Baseline Period (Day − 1), and was followed by a Treatment Phase consisting of a 35-day Treatment Period (Days 1–35). Subjects received once-daily oral doses of perampanel 2 mg (one 2-mg tablet) on Days 1–7 and once-daily oral doses of perampanel 4 mg (two 2-mg tablets) on Days 8–21. Perampanel was administered under fasted conditions (≥ 10 h overnight) on Days 1 and 21, and under fed conditions on all other days. Subjects were confined to the study site on Days − 1 to 26, and had follow-up outpatient visits on Days 30 and 35. Subjects were financially compensated for the temporary loss of working time when confined to study sites as described in the informed consent form.
2.4 Pharmacokinetic Assessments
2.4.1 Sample Collection
Blood samples for pharmacokinetic analysis (4 mL each) were collected on Days 1–15 (pre-dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, 96, 168, 240, and 336 h post-dose) for single-dose administration, and on Days 1 and 21 (pre-dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 12 h post-dose on both days), and Days 22, 23, 24, 26, 30, and 35 (i.e., 24, 48, 72, 120, 216, and 336 h after dosing on Day 21) for multiple-dose administration. Collected blood samples were centrifuged at 2000×g for 15 min at room temperature, and the supernatant (plasma) was separated and kept in frozen condition until analysis.
2.4.2 Bioanalytical Assay
As previously described, the plasma concentrations of perampanel were measured using a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS). Concentration measurements were made in the range of 1.00–500 ng/mL [15, 16].
A perampanel analog, ER-167615, was used as the internal standard (IS) for the analysis. The lower limit of quantification was set at 1.00 ng/mL using 100 μL of plasma. Samples above the upper limit of quantification were diluted tenfold using blank plasma. All plasma samples were analyzed with quality control samples (low, mid, and high concentrations) and all assay batches met the acceptance criteria (≤ 15% for at least 2/3 samples, and at least 50% of each concentration level).
The high-performance liquid chromatography system used Shimadzu 20A series (Online Degasser DGU-20A5; LC-20AD pumps; SIL-20AC autosampler/SIL-20AC autosampler with Rack Change II; CTO-20A Column Oven) coupled with a triple quadrupole mass spectrometer API4000 (AB Sciex) in the positive ion electrospray ionization mode. Selected precursor and product ion transitions of m/z 350 → 219 and m/z 359 → 323 were monitored for the detection of perampanel and the IS, respectively. Plasma samples were extracted by protein precipitation with methanol followed by centrifugation to remove protein. The supernatant was injected directly for the LC-MS/MS analysis. Reverse-phase high-performance liquid chromatography utilized Unison UK-C8 column (4.6×50 mm, 3 μm particle; Intact Corporation). Perampanel and IS were eluted with isocratic elution of mobile phase comprising 0.1% formic acid and 2.0 mmol/L ammonium formate in methanol/water (75:25, v/v) at a flow rate of 1.0 mL/min. Quantitation was achieved based on a 1/x2 weighted regression analysis of the peak area ratios of perampanel/IS versus concentrations.
2.4.3 Pharmacokinetic Analysis
Pharmacokinetic analyses were performed on the Pharmacokinetic Analysis Set, which comprised all subjects who received study drug and had sufficient pharmacokinetic data to derive at least one pharmacokinetic parameter. Pharmacokinetic parameters of perampanel were estimated using noncompartmental methods with Phoenix WinNonlin™ version 7.0 (Certara USA, Inc., Princeton, NJ, USA). SAS® version 9.4 was used for all statistical analyses (SAS Institute Inc., Cary, NC, USA). The statistical analysis method for this study is descriptive in nature. Plasma concentrations of perampanel were tabulated by nominal sampling time and summarized by dose using summary statistics. Plasma concentration–time profiles were plotted.
Pharmacokinetic parameters evaluated for single-dose administration included Cmax, time at which Cmax occurred (tmax), area under the concentration–time curve from time zero to time of the last quantifiable concentration (AUC(0-t)), area under the concentration–time curve from time zero extrapolated to infinite time (AUC(0-inf)), terminal elimination phase half-life (t½), apparent total clearance following oral administration (CL/F), and apparent volume of distribution at the terminal phase (Vz/F).
Dose proportionality for Cmax, AUC(0– t), and AUC(0-∞) following single-dose administration was evaluated using a linear model of logarithmically transformed values to assess the slope parameter and 95% confidential intervals (CIs), and was evaluated graphically. Statistical analyses were performed on natural log-transformed data using a mixed-effect model including terms for intercept and slope for natural log dose level (added as a numeric covariate), with subject as a random effect.
Pharmacokinetic parameters evaluated for multiple-dose administration included Cmax, minimum observed concentration (Cmin), tmax, and AUC(0-24h), which were evaluated on Day 1, and area under the concentration–time curve over the dosing interval on multiple dosing (AUC(0–τ)), average steady-state concentration (Css,av), maximum observed concentration at steady state (Css,max), time at which the highest drug concentration occurs at steady state (tss,max), t½, apparent total clearance following oral administration at steady state (CLss/F), Vz/F, accumulation ratio of AUC (Rac [AUC], calculated as AUC(0–τ) on Day 21 / [AUC(0-24h) on Day 1 × 2]), and accumulation ratio of Cmax (Rac [Cmax], calculated as Cmax on Day 21/[Cmax on Day 1 × 2]) were evaluated on Day 21.
The weight-adjusted Css,max was calculated as Css,max × body weight for each subject (kg) / 70 (kg), and weight-adjusted AUC(0-τ) was calculated as AUC(0-τ)) × body weight for each subject (kg) / 70 (kg). These parameters were calculated to permit comparison with other populations from previously published work.
2.5 Safety Assessments
Safety analyses were performed on the Safety Analysis Set, which comprised all subjects who received study drug and had one or more post-dose safety assessments. Safety was evaluated via the monitoring of adverse events (AEs), serious AEs (SAEs), laboratory evaluations (including hematology, chemistry, and urinalysis), and vital signs, as well as performing physical examinations and 12-lead electrocardiogram (ECG) recordings throughout the single- and multiple-dose parts of the study.
AEs included any unfavorable and unintended sign, symptom, or disease temporally associated with the use of study drug, regardless of causality; any new disease or exacerbation of an existing disease; any deterioration in non-protocol-required measurements of a laboratory value or other clinical test that results in symptoms, a change in treatment, or discontinuation from study drug; and recurrence of an intermittent medical condition not present during baseline. An abnormal laboratory test result was considered an AE if the identified laboratory abnormality led to any type of intervention, discontinuation from study drug, or withholding of study drug, whether prescribed in the protocol or not. AEs were graded on a 3-point scale (mild, moderate, and severe). SAEs included any AEs that were life-threatening at any dose, resulted in death or persistent or significant disability/incapacity, required inpatient hospitalization or prolongation of existing hospitalization, or were a congenital anomaly/birth defect.
3 Results
3.1 Single-Dose Administration
3.1.1 Subjects
Thirty-two subjects were enrolled in the single-dose part of the study. Two subjects discontinued the study prior to treatment administration and did not receive perampanel. Overall, 30 subjects completed the study and were included in the Pharmacokinetic Analysis Set and Safety Analysis Set (10 subjects in each of the 2-, 4-, and 8-mg dose groups). Baseline demographics are provided in Table 1.
3.1.2 Pharmacokinetic Analysis
The mean plasma concentration–time profiles following a single oral dose of perampanel 2, 4, or 8 mg are shown in Fig. 1, and the corresponding pharmacokinetic parameters are summarized in Table 2. Mean perampanel Cmax ranged from 77.9 ng/mL for perampanel 2 mg to 276 ng/mL for perampanel 8 mg. Mean AUC(0-t) and AUC(0-inf) were, respectively, 4070 and 5040 ng∙h/mL for perampanel 2 mg, 7740 and 8770 ng∙h/mL for perampanel 4 mg, and 15,100 and 17,000 ng∙h/mL for perampanel 8 mg. Perampanel was rapidly absorbed, with a median tmax of 0.75–1.0 h, and slowly eliminated (mean t½ = 85.6–122 h).
Evaluation of Cmax, AUC(0-t), and AUC(0-inf) for dose proportionality revealed a slope β (95% CI) of 0.909 (0.787–1.031) for Cmax, 0.932 (0.718–1.145) for AUC(0-t), and 0.873 (0.606–1.140) for AUC(0-inf). As the 95% CIs for Cmax, AUC(0-t), and AUC(0-inf) included 1, and tmax, t½, CL/F, and Vz/F remained relatively constant (Table 2), it was concluded that the pharmacokinetics of perampanel were linear across the dose range investigated in this study (2–8 mg).
3.2 Multiple-Dose Administration
3.2.1 Subjects
Twelve subjects were enrolled, administered, and completed the study. All 12 subjects were included in the Pharmacokinetic Analysis Set and Safety Analysis Set. One subject experienced vomiting 12 h after dosing on Day 14 and 8 h after dosing on Day 15. Since both instances of vomiting occurred after a duration that was at least six times the median multiple-dose tmax (1.25 h), the data for this subject were included in the pharmacokinetic analysis.
3.2.2 Pharmacokinetic Analysis
The mean plasma concentration–time profiles on the first (Day 1) and last (Day 21) days of multiple dosing of perampanel are shown in Fig. 2, and the corresponding pharmacokinetic parameters are summarized in Table 3. On Day 1 (perampanel 2 mg), mean Cmax was 82.2 ng/mL, mean AUC(0-24h) was 667 ng∙h/mL, and median tmax was 0.75 h. On Day 21 at steady-state perampanel 4-mg dosing, mean Css,max was 453 ng/mL, mean AUC(0-τ) was 7540 ng∙h/mL, median tss,max was 1.25 h, and mean t½ was 109 h. The accumulation ratios Rac (Cmax) and Rac (AUC) were 2.93 and 6.03, respectively, indicating that perampanel accumulated with repeated administration.
The mean (standard deviation [SD]) weight-adjusted Css,max and weight-adjusted AUC(0-τ) in Chinese subjects were 401 (145) ng/mL and 6680 (2450) ng∙h/mL, respectively (Table 3, Fig. 3). No obvious differences in weight-adjusted Css,max and weight-adjusted AUC(0-τ) between Chinese females and males were apparent visually (Fig. 3), thus a statistical analysis was not conducted.
Comparison of a weight-adjusted Css,max, and b weight-adjusted AUC(0-τ) in healthy Chinese, White, Japanese, and Korean subjects receiving perampanel 4 mg/day at steady statea. Non-weight-adjusted data for White, Japanese, and Korean subjects have been published previously by Tabuchi et al. [13]
3.3 Safety Analysis
An overview of the AEs reported in the single- and multiple-dose studies is provided in Table 4. Treatment-emergent AEs (TEAEs) were reported by 21/30 (70.0%) subjects in the single-dose part and 11/12 (91.7%) subjects in the multiple-dose part. All TEAEs, other than in one subject with increased neutrophil count, were deemed by the investigator to be treatment-related. There were no serious TEAEs, deaths, or discontinuations due to TEAEs in either part of the study. Other than one instance of white blood cells in urine, which was described as moderate in intensity, all TEAEs were mild in intensity. The most common TEAEs were dizziness and somnolence (Table 4).
Overall, six subjects had clinically significant laboratory abnormalities, all of which were reported as TEAEs. One subject each experienced an increase in blood triglycerides, a decrease in hemoglobin, an increase in blood potassium, an increase in neutrophil count, an instance of white blood cells in the urine, and bacteriuria. No clinically significant changes in vital signs and ECG were reported.
4 Discussion
This study investigated the pharmacokinetics of single- and multiple-dose perampanel in healthy Chinese adults. Single doses of perampanel 2, 4, or 8 mg were rapidly absorbed (median tmax 0.75–1.0 h post-dose) and slow elimination was observed (mean t½ = 85.6–122 h). Single-dose perampanel pharmacokinetics were found to be linear over the investigated dose range of 2–8 mg. These results are consistent with what has been published previously regarding the single-dose pharmacokinetics with perampanel tablets over a dose range of 1–12 mg/day (median tmax 1.0 h; mean t½ = 85.2–117.4 h) for other populations, including single-dose perampanel pharmacokinetics being linear [11].
Similar to the single-dose pharmacokinetics, evaluation of perampanel at steady-state following multiple daily dosing with perampanel 4 mg revealed slow elimination, with a mean t½ of 109 h, and fast absorption, with a median tmax of 1.25 h. Evaluation of the accumulation ratios on Day 21 (Rac [Cmax] 2.93; Rac [AUC] 6.03) also suggested that drug accumulation was evident with repeated administration of perampanel. Steady-state perampanel pharmacokinetics have been previously published for healthy White, Japanese, and Korean subjects [13]. Values for the time at which the highest drug concentration occurs at steady state (tmax,ss) and t½ were comparable between groups (median tmax,ss: 1.25 h for Chinese subjects versus 1 h for White, Japanese, and Korean subjects; mean [SD] t½: 109 [46.7] h for Chinese subjects, 129 [146] h for White subjects, 63.9 [30.0] h for Japanese subjects, and 105 [34.3] h for Korean subjects [13]). The mean (SD) Css,max following multiple perampanel 4 mg dosing was slightly higher for Chinese subjects (453 [155] ng/mL) compared with White subjects, but comparable with Japanese and Korean subjects (365 [78.7], 433 [127], and 430 [129] ng/mL, respectively) [13]. Once differences in body weight were accounted for, Css,max for Chinese subjects was also comparable with that for the White population, as shown in Fig. 3 (mean [SD] weight-adjusted Css,max: Chinese, 401 [145] ng/mL; White, 404 [95.0] ng/mL; Japanese, 400 [135] ng/mL; Korean, 434 [99.9] ng∙h/mL [13]). Similarly, the mean (SD) AUC(0- τ) after multiple dosing with perampanel 4 mg was also slightly higher in Chinese subjects (7540 [2620] ng∙h/mL) versus White subjects (6200 [1770] ng∙h/mL), and comparable with Japanese and Korean subjects (6840 [2290] and 7690 [2380] ng∙h/mL, respectively). After weight adjustment, the mean [SD] AUC(0- τ) in Chinese subjects became analogous to White subjects, but slightly lower than Korean subjects (Fig. 3; mean [SD] weight-adjusted AUC(0- τ): Chinese, 6680 [2450] ng∙h/mL; White, 6900 [2210] ng∙h/mL; Japanese, 6340 [2410] ng∙h/mL; Korean, 7780 [1880] ng∙h/mL [13]). As seen in Fig. 3, interindividual variability of weight-adjusted AUC(0- τ) and weight-adjusted Css,max within each group was large, and the distribution of these pharmacokinetic parameters was largely overlapped and similar among Chinese, White, Japanese, and Korean subjects. As the difference in mean AUC(0- τ) was < 20% compared with other populations, we concluded that results were comparable with those reported previously for White, Japanese, and Korean subjects.
These findings are also consistent with a previous population-based pharmacokinetic study of perampanel in patients with focal-onset seizures, which included Chinese patients [14]. It found that Japanese or Chinese race had no impact on CL/F or on the probability of a response to perampanel [14].
Based on the data presented in this study relative to other known multiple-dose pharmacokinetic studies, there were no clinically significant differences in multiple-dose perampanel pharmacokinetics between Chinese adults and previously studied healthy populations of different races [14]. As such, the previously established dosing regimens for perampanel are applicable to Chinese patients without the need for dose adjustment.
For both single- and multiple-dose perampanel, perampanel was found to be generally safe and well tolerated. There were no serious TEAEs, deaths, or study drug withdrawals due to TEAEs, and all TEAEs except one instance of white blood cells in urine were mild in intensity. The most common TEAEs were dizziness and somnolence, which are consistent with the most commonly reported TEAEs during phase III studies [5,6,7,8,9,10].
This study has the limitation of being carried out in healthy subjects, as opposed to in patients with epilepsy. Although this study was performed in healthy individuals, the findings are expected to be applicable to patients with focal-onset seizures and/or generalized tonic-clonic seizures based on previous findings that pharmacokinetics are similar between these different populations [3].
5 Conclusions
In summary, a study of single- and multiple-dose pharmacokinetics of perampanel in healthy Chinese adults revealed rapid perampanel absorption, as indicated by a short tmax, and slow elimination, as indicated by a long t½. Perampanel single-dose pharmacokinetics were also shown to be linear with perampanel doses of 2–8 mg. At steady state, drug accumulation was evident following repeated administration of perampanel. Single and multiple doses of perampanel were generally safe and well tolerated, and the most commonly reported TEAEs of dizziness and somnolence were in line with those observed in previous studies.
Notes
WA AUC(0-τ) weight-adjusted AUC(0-τ), WA Css,max weight-adjusted Css,max, Bar indicates the median. a
References
Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, et al. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52:1331–40. https://doi.org/10.1111/j.1528-1167.2011.03109.x.
European Medicines Agency (EMA). FYCOMPA® Annex I: Summary of Product Characteristics. August 2022. https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf. Accessed 10 Aug 2022.
US Food and Drug Administration (FDA). FYCOMPA® prescribing information. December 2021. https://www.fycompa.com/-/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed 28 Feb 2022.
Eisai Co., Ltd. Anti-epileptic drug Fycompa® approved in China as monotherapy for partial-onset seizures and pediatric indication for partial-onset seizures. 2021. https://www.eisai.com/news/2021/news202166.html. Accessed 5 May 2022.
French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: Randomized phase III study 304. Neurology. 2012;79:589–96. https://doi.org/10.1212/WNL.0b013e3182635735.
French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–25. https://doi.org/10.1111/j.1528-1167.2012.03638.x.
Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–15. https://doi.org/10.1212/WNL.0b013e318254473a.
Nishida T, Lee SK, Inoue Y, Saeki K, Ishikawa K, Kaneko S. Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study. Acta Neurol Scand. 2018;137:392–9. https://doi.org/10.1111/ane.12883.
Krauss GL, Perucca E, Kwan P, Ben-Menachem E, Wang X-F, Shih JJ, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open-label extension of phase III randomized trials: study 307. Epilepsia. 2018;59:866–76. https://doi.org/10.1111/epi.14044.
French JA, Krauss GL, Wechsler RT, Wang X-F, DiVentura B, Brandt C, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology. 2015;85:950–7. https://doi.org/10.1212/WNL.0000000000001930.
Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56:12–27. https://doi.org/10.1111/epi.12865.
Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23. https://doi.org/10.1038/clpt.2008.141.
Tabuchi H, Shiba S, Yasuda S, Ohnishi A, Shin JG. Pharmacokinetics of perampanel in healthy Korean, White, and Japanese Adult Subjects. Clin Pharmacol Drug Dev. 2018;7:613–20. https://doi.org/10.1002/cpdd.581.
Takenaka O, Ferry J, Saeki K, Laurenza A. Pharmacokinetic/pharmacodynamic analysis of adjunctive perampanel in subjects with partial-onset seizures. Acta Neurol Scand. 2018;137:400–8. https://doi.org/10.1111/ane.12874.
Mano Y, Takenaka O, Kusano K. High-performance liquid chromatography-tandem mass spectrometry method for the determination of perampanel, a novel a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist in human plasma. J Pharm Biomed Anal. 2015;107:56–62. https://doi.org/10.1016/j.jpba.2014.12.018.
Mano Y. An inter-laboratory cross-validation study for the determination of perampanel in human plasma by liquid chromatography assays. Biomed Chromatogr. 2016;30:2067–9. https://doi.org/10.1002/bmc.3764.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Kirsty Muirhead, PhD, of CMC AFFINITY, McCann Health Medical Communications, funded by Eisai Inc., in accordance with Good Publication Practice (GPP 2022) guidelines. The authors would like to thank Kohei Ishikawa, Hidetaka Hiramatsu, and Kenya Nakai, all of whom are employees of Eisai Co., Ltd, who reviewed this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eisai Co., Ltd.
Conflicts of Interest
Shan Jing and Yang Lin declare that they have no conflicts of interest to disclose. Sari Shiba, Masafumi Morita, and Sanae Yasuda are employees of Eisai Co., Ltd.
Ethics approval
The study protocol and informed consent forms were reviewed and approved by the Institutional Review Board of Beijing Anzhen Hospital, China.
Consent to participate
All participants provided written informed consent before participation in the study and after the study procedures had been fully explained.
Consent to publish
The informed consent form included a disclaimer that data will be anonymized and published.
Availability of data and material
The data are not available in a repository but reasonable requests can be directed to the corresponding author at ICP@anzhengcp.com.
Code availability
Not applicable.
Author Contributions
Study conception and/or design: SJ, SS, SY. Data acquisition: SJ, YL, MM. Data analysis: SJ, SS, SY. Interpretation of results: SJ, YL, MM, SS, SY. All authors were involved in the review and approval of the manuscript, and in the decision to submit the article for publication. All authors also confirm accountability for the accuracy and integrity of the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jing, S., Shiba, S., Morita, M. et al. A Single- and Multiple-Dose Pharmacokinetic Study of Oral Perampanel in Healthy Chinese Subjects. Clin Drug Investig 43, 155–165 (2023). https://doi.org/10.1007/s40261-022-01241-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01241-8