Abstract
Background
Canakinumab is a human anti-interleukin-1beta antibody approved for the treatment of cryopyrin associated periodic syndrome currently formulated as a lyophilized powder requiring reconstitution. A new formulation (solution for injection as pre-filled syringe) has been developed to avoid reconstitution.
Objective
The objective of this study was to evaluate the bioequivalence of pre-filled syringe and reconstituted formulations following 150 mg administration in healthy subjects.
Methods
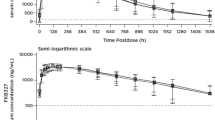
This was an open-labeled, randomized, single dose, parallel-group study in 130 healthy subjects, followed for 120 days. Subjects received a single subcutaneous injection of 150 mg canakinumab after either reconstitution or in pre-filled syringe formulation, followed by pharmacokinetics/pharmacodynamics evaluations and safety assessments. The main outcome measure for the study was the pharmacokinetic bioequivalence of the two formulations, which was concluded if the 90 % confidence intervals for the ratios of AUClast (area under the serum concentration-time curve from time zero to time of last measurable concentration) and C max (maximum serum concentration) were entirely contained within the interval, 0.80–1.25.
Results
The arithmetic mean values for the exposure parameters C max and AUClast were similar for the two formulations. The geometric mean ratio (pre-filled syringe vs. lyophilized form) of C max and AUClast were 0.99 and 1.01. The associated 90 % confidence intervals were 0.90 to 1.08 and 0.94 to 1.09, respectively. Most common adverse events were headache and nasopharyngitis. Neutropenia occurred in 2 cases (reported as serious adverse events). No deaths occurred.
Conclusion
The 150 mg liquid pre-filled syringe and lyophilized formulations of canakinumab are bioequivalent.



Similar content being viewed by others
References
Chakraborty A, Tannenbaum S, Rordorf C, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1–18.
Hoffman HM, Wanderer AA. Inflammasome and IL-1β-mediated disorders. Curr Allergy Asthma Rep. 2010;10:229–35.
Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–41.
Ilaris® packaging insert. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/001109/WC500031680.pdf. Accessed 27 Jun 2013.
Specifications: Test procedures and acceptance criteria for biotechnological/biological products Q6B, ICH harmonised tripartite guideline. 1999. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q6B/Step4/Q6B_Guideline.pdf. Accessed 27 Jun 2013.
Guidance for Industry. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products-General Considerations, Rockville: CDER2006. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf. Accessed 27 Jun 2013.
Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146(7):486–92.
Acknowledgments
Andrea Chioato, Emanuele Noseda, Laurence Colin, Ralph Matott, Andrej Skerjanec, Albert J. Dietz are employees of Novartis Pharma AG, Switzerland and have participated in the preparation of the manuscript. This study and the manuscript were funded by Novartis Pharma AG, Switzerland.
Conflict of interest
A. Chioato and E. Noseda are Novartis employee. A. Skerjanec is a employee of Novartis. L. Colin is at Novartis Pharma AG. R. Matott work for the sponsor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chioato, A., Noseda, E., Colin, L. et al. Bioequivalence of Canakinumab Liquid Pre-Filled Syringe and Reconstituted Lyophilized Formulations Following 150 mg Subcutaneous Administration: A Randomized Study In Healthy Subjects. Clin Drug Investig 33, 801–808 (2013). https://doi.org/10.1007/s40261-013-0127-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0127-4