Abstract
Background and Objective
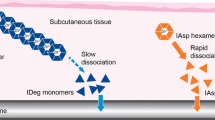
Insulin degludec (IDeg) is a new-generation basal insulin that forms soluble multi-hexamers upon subcutaneous injection, resulting in a depot from which IDeg monomers are slowly and continuously absorbed to provide an ultra-long action profile. This double-blind, crossover, randomized study compared the pharmacokinetic and pharmacodynamic properties between IDeg 100 U/mL (U100) and IDeg 200 U/mL (U200) under steady-state (SS) conditions in subjects with type 1 diabetes mellitus.
Methods
Participants (n = 33 adults) underwent 8-day treatment periods with 0.4 U/kg IDeg U100 and IDeg U200 given once daily with insulin aspart at mealtimes. On day 8, a 26-h euglycaemic glucose clamp (5.5 mmol/L) was performed.
Results
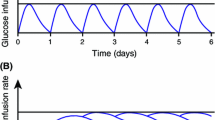
The concentration–time profiles of IDeg U100 and IDeg U200 were similar, and a post-hoc analysis showed bioequivalence between these formulations, as the 90 % confidence intervals (CIs) of the U200/U100 ratios for area under the steady-state serum IDeg concentration-time curve during a dosing interval (τ; 0–24 h) (AUCτ,SS,IDeg) (0.99 [0.91–1.07]) and maximum steady-state IDeg concentration during a dosing interval (τ) (C max,SS,IDeg) (0.93 [0.84–1.02]) were within the interval 0.80–1.25. Comparable glucose infusion rates (GIR) were observed for IDeg U100 and IDeg U200 (AUCτ,SS,GIR [mg/kg]: 2,255 vs. 2,123) and the mean ratio (95 % CI) of IDeg U200/U100 for the primary endpoint (AUCτ,SS,GIR) was 0.94 [0.86–1.03]. For both formulations, the glucose-lowering effect of IDeg was evenly distributed between the first and second 12 h post-dosing (U100: AUC12,SS,GIR/AUC24,SS,GIR = 48 %; U200: AUC12,SS,GIR/AUC24,SS,GIR = 46 %). Both formulations were well tolerated, and no safety events of significance were identified.
Conclusion
IDeg U100 and U200 formulations are bioequivalent and have similar pharmacodynamic profiles at SS, implying that they can be used interchangeably in clinical practice.


Similar content being viewed by others
References
Heller S, Koenen C, Bode B. Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin Ther. 2009;31:2086–97.
Hamann A, Matthaei S, Rosak C, Silvestre L. A randomized clinical trial comparing breakfast, dinner, or bedtime administration of insulin glargine in patients with type 1 diabetes. Diabetes Care. 2003;26:1738–44.
Albright ES, Desmond R, Bell DSH. Efficacy of conversion from bedtime NPH insulin injection to once- or twice-daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy. Diabetes Care. 2004;27:632–3.
Kampmann U, Hoeyem P, Mengel A, et al. Insulin dose-response studies in severely insulin-resistant type 2 diabetes: evidence for effectiveness of very high insulin doses. Diabetes Obes Metab. 2011;13:511–6.
Jonassen I, Havelund S, Hoeg-Jensen T, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29:2104–14.
Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64.
Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–50.
Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Mathieu C, Hollander P, Miranda-Palma B, Cooper J, Franek E, Russell-Jones D, Larsen J, Tamer SC, Bain SC, NN1250-3770 (BEGIN: Flex T1) Trial Investigators. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–62.
Meneghini L, Atkin SL, Gough SC, Raz I, Blonde L, Shestakova M, Bain S, Johansen T, Begtrup K, Birkeland KI, NN1250-3668 (BEGIN FLEX) Trial Investigators. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–64.
International Conference on Harmonisation. ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1), Step 4. 10 June 1996.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Last amended by the 59th WMA General Assembly, Seoul, 2008.
ADA—American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005; 28(5):1245–49.
EMEA: Committee for Medical Products for Human use (CHMP) CPMP/EWP/QWP/1401/98 Rev. 1—Guideline on the investigation of bioequivalence. http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
Rodbard H, Handelsman Y, Gough S, et al. Reduced risk of hypoglycemia with insulin degludec vs insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: meta-analysis of five randomized trials. American Association of Clinical Endocrinologists 21st Annual Scientific Meeting and Clinical Congress 2012 [Abstract 241].
Bergenstal R, Bhargava A, Jain R, et al. Improved patient-reported outcomes with insulin degludec 200 U/mL (IDeg U200) versus insulin glargine in insulin-naïve people with type 2 diabetes. American Association of Clinical Endocrinologists 21st Annual Scientific Meeting and Clinical Congress 2012 [Abstract 69].
Bergenstal R, Bhargava A, Jain R, et al. 200 U/mL insulin degludec improves glycemic control similar to insulin glargine with a low risk of hypoglycemia in insulin-naïve people with type 2 diabetes. American Association of Clinical Endocrinologists 21st Annual Scientific Meeting and Clinical Congress 2012 [Abstract 26].
Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464–71.
Funding
This study was supported financially by Novo Nordisk A/S, Denmark, which was also responsible for the design, analysis and reporting of the study, with input from the authors. All authors were involved in the preparation and approval of the manuscript in collaboration with Novo Nordisk.
Acknowledgments
The authors thank all the subjects who participated in the study. The authors also thank Irene Vejgaard Sørensen (Novo Nordisk A/S) for assistance in preparing this paper and Mark Nelson (Watermeadow Medical, sponsored by Novo Nordisk) for assisting with the preparation of figures and tables and the submission of this article.
Conflicts of Interest
SD and KN have had no support from any organization for the submitted work; SK had support from Novo Nordisk A/S for the submitted work; SK, JKM, GK and TRP have received fees for speaking and/or consulting from Novo Nordisk A/S in the previous 3 years; CA, HT and HH are employed by and hold stock in Novo Nordisk A/S; no other relationships or activities influenced the submitted work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov identifier: NCT01076634.
Rights and permissions
About this article
Cite this article
Korsatko, S., Deller, S., Koehler, G. et al. A Comparison of the Steady-State Pharmacokinetic and Pharmacodynamic Profiles of 100 and 200 U/mL Formulations of Ultra-Long-Acting Insulin Degludec. Clin Drug Investig 33, 515–521 (2013). https://doi.org/10.1007/s40261-013-0096-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0096-7