Abstract
Background and Objectives
The burden of type 2 diabetes mellitus is growing rapidly, particularly in the Asia-Pacific region. The aim of this international, large-scale, observational study was to investigate the efficacy and tolerability of the antidiabetic agent acarbose as add-on or monotherapy in a range of patients with type 2 diabetes, including those with cardiovascular morbidities. The majority of practices were included from high-burden regions (predominantly those in the Asia-Pacific region).
Methods
This was an observational study conducted in 15 countries/regions. Adults with pre-treated or untreated type 2 diabetes prescribed acarbose as add-on or monotherapy were eligible. Two-hour postprandial blood glucose (2-h PPG), glycosylated haemoglobin (HbA1c) and fasting blood glucose (FBG) were measured over a 3-month observation period.
Results
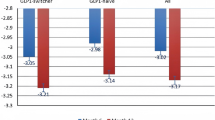
A total of 15,034 patients were valid for the efficacy analysis and 15,661 for the safety analysis (mean age was 57.6 years and 92.6 % of patients were Asian). Mean (SD) 2-h PPG decreased by −71.9 (62.3) mg/dL, to 170.2 (46.5) mg/dL at final visit (after 12.8 [4.1] weeks). Mean HbA1c decreased by −1.1 % (1.3) to 7.2 % (1.1) and mean FBG decreased by −33.0 (43.3) mg/dL to 124.8 (30.5) mg/dL. Acarbose was effective regardless of the presence of cardiovascular co-morbidities or diabetic complications. The efficacy of acarbose was rated ‘very good’ or ‘good’ in 85.5 % of patients, and tolerability as ‘very good’ or ‘good’ in 84.9 % of patients. Drug-related adverse events, mainly gastrointestinal, were reported in 490/15,661 patients (3.13 %).
Conclusion
The results of this observational study support the notion that acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes.




Similar content being viewed by others
References
IDF Diabetes Atlas. International Diabetes Federation. 5th ed. http://www.idf.org/diabetesatlas/5e/the-global-burden. Accessed 5 Jan 2012.
Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28(9):2130–5.
Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342(6):381–9.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53.
Liu Z, Fu C, Wang W, et al. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients—a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010;8:62.
Choi YJ, Kim HC, Kim HM, et al. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care. 2009;32(11):2016–20.
Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med. 2007;24(5):451–63.
Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39(12):1577–83.
DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397–405.
Meigs JB, Nathan DM, D’Agostino RB Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25(10):1845–50.
Levitan EB, Song Y, Ford ES, et al. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–55.
Nakagami T, DECODA Study Group. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47(3):385–94.
Monnier L, Colette C, Boniface H. Contribution of postprandial glucose to chronic hyperglycaemia: from the “glucose triad” to the trilogy of “sevens”. Diabetes Metab. 2006;32(Spec. No 2):2S11–6.
Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77(2):280–5.
Peter R, Okoseime OE, Rees A, et al. Postprandial glucose—a potential therapeutic target to reduce cardiovascular mortality. Curr Vasc Pharmacol. 2009;7(1):68–74.
Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84.
Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164(19):2090–5.
2011 Guideline for Management of PostMeal Glucose in Diabetes. International Diabetes Federation. http://www.idf.org/sites/default/files/postmeal%20glucose%20guidelines.pdf. Accessed 3 Feb 2011.
Derosa G, Maffioli P. Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review. Clin Ther. 2012;34:1221–36.
DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617–21.
The DECODA Study Group. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47:385–94.
Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34(10):2237–43.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881–5.
Spengler M, Schmitz H, Landen H. Evaluation of the efficacy and tolerability of acarbose in patients with diabetes mellitus : a postmarketing surveillance study. Clin Drug Investig. 2005;25(10):651–9.
Mertes G. Safety and efficacy of acarbose in the treatment of Type 2 diabetes: data from a 5-year surveillance study. Diabetes Res Clin Pract. 2001;52(3):193–204.
Li C, Hung YJ, Qamruddin K, et al. International noninterventional study of acarbose treatment in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;92(1):57–64.
Derosa G, Maffioli P, Ferrari I, et al. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur J Pharmacol. 2011;651(1–3):240–50.
Derosa G, Maffiolo P, D’Angelo A, et al. Acarbose on insulin resistance after an oral fat load: a double-blind, placebo controlled study. J Diabetes Complicat. 2011;25(4):258–66.
Derosa G, Salvadeo SA, D’Angelo A, et al. Metabolic effect of repaglinide or acarbose when added to a double oral antidiabetic treatment with sulphonylureas and metformin: a double-blind, cross-over, clinical trial. Curr Med Res Opin. 2009;25(3):607–15.
Derosa G, D’Angelo A, Salvadeo SA, et al. Modulation of adipokines and vascular remodelling markers during OGTT with acarbose or pioglitazone treatment. Biomed Pharmacother. 2009;63(10):723–33.
Derosa G, Mereu R, D’Angelo A, et al. Effect of pioglitazone and acarbose on endothelial inflammation biomarkers during oral glucose tolerance test in diabetic patients treated with sulphonylureas and metformin. J Clin Pharm Ther. 2010;35(5):565–79.
American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11–61.
Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen Study. Diabetes Care. 1994;17(6):561–6.
Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study. Am J Med. 1997;103(6):483–90.
Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25(4):435–41.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–7.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Chiasson JL. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr Pract. 2006;12(Suppl 1):25–30.
Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25(1):10–6.
Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol. 2007;6:20.
Spengler M, Cagatay M. The use of acarbose in the primary-care setting: evaluation of efficacy and tolerability of acarbose by postmarketing surveillance study. Clin Invest Med. 1995;18(4):325–31.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358(24):2630–3.
Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111(7):405–14.
Fach information: Glucobay 50 mg, Glucobay 100 mg. 2010. Document 003443-D671 DE/28.
Acknowledgements
This work was funded by a research grant from Bayer, Berlin, Germany. Editorial assistance was provided by PAREXEL, which was contracted by Bayer, Berlin, Germany.
Author Contributions
Weiwei Zhang, DongJun Kim, Elizabeth Philip, Zahid Miyan and Irina Barykina were involved in data collection and analysis, and were involved in writing and reviewing the manuscript. Herbert Stein and Birgit Schmidt were involved in the study design, data collection and analysis, and writing and reviewing the manuscript.
Conflict of interest
Herbert Stein and Birgit Schmidt are employees of Bayer HealthCare, Germany. No other authors report a conflict of interest.
Role of the Funding Source
Bayer were involved in the study design, the collection, analysis and interpretation of data, and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the GlucoVIP Investigators.
The GlucoVIP investigators are given in the Appendix.
Appendix: Contributing Investigators
Appendix: Contributing Investigators
Abbass, Z; Abdus Sattar, J; Abdurahman, T; Abid, R; Adhiwana, S; Adi Utama, L; Agarwal, R; Ahmad, BI; Ahmad, S; Ahmad, M; Ahmed, B; Ahmed, R; Ahmed, U; Ahmed, M; Ahn, C; Ahn, C; Ahn, J; Ahn, K; Akbani, S; Akhter, A; Akram, M; Alajbegovic, S; Alam, R; Alampayan, M; Ali, S; Ali, M; Ali Awan, M; Ali Choudhary, I; Ali Shaikh, A; Ali Sheikh, K; Alip, M; Alphanto, W; Alves, J; Ambhore, S; Aminwala, M; An, M; Andriani, L; Andag-Silva, A; Ang, A; Anhua, L; Antonio, A; Aouali, R; Aouiche, S; Apit, S; Aquino, J; Arain, M; Arifiyanto, I; Ashraf, B; Ashraf, F; Atmosutarjo, S; Au, C; Auliati, S; Awan, H; Babakaeva, A; Babbar, P; Bachtiarwati, A; Baculao, E; Bagiyan, I; Bai, Y; Baik, T; Baloch, F; Baldemor, D; Banerjee, J; Barykina, I; Basri, H; Batavia, I; Bavishi, R; Benazzouz, M; Bespalova, T; Bey, A; Bhar, P; Bhattacharya, S; Bien, T; Bin, W; Borovcanin, D; Bong, C; Bongirwar, V; Bova, E; Bullo, J; Butar-Butar, R; Butt, J; Cao, A; Cao, H; Carpio, B; Carreon, R; Ceric, K; Cha, S; Chagay, N; Chajjer, K; Chan, P; Chan, D; Chan, N; Chan, N; Chan, W; Chandramohan, P; Chandra, S; Chang, K; Chao, X; Chauhan, S; Chea, T; Cheng, H; Cheong, A; Cheung, K; Chey, S; Chi, K; Chia, Y; Chin, Y; Chiu, K; Cho, E; Cho, H; Cho, YW; Choi, BK; Choi, BI; Choi, DH; Choi, DS; Choi, I; Choi, S; Choi, WS; Chong, L; Chua, A; Chun, HC; Chun, R; Chung, C; Chung, J; Chung SC; Cimafranca, A; Crisostomo, M; Cui, C; Custodio, M; Cutillar, C; Dabir, H; Darmawi, S; Darwis, D; Das, R; Datta, K; Dela Cruz, T; Deng, W; Deole, M; Deshpande, S; Devan, KS; Dewanti, D; Dewi, M; Dilber, S; Dimatatac, G; Dirjayanto, A; Dizdarevic, A; Djukanovic, Z; Dris, I; Duero, D; Dudareva, L; Duong Tan, T; Echavia, A; Egorova, V; Escalante, V; Esparagoza, C; Eugenio, B; Evuru, S; Fababier, R; Farooq, D; Farooqi, D; Fatimah, F; Fei, T; Feng, Z; Fengming, Z; Filatova, O; Florece, M; Fomina, S; Fu, Q; Fuaad, M; Gafate-Ong, M; Gamallo, E; Gan, M; Gang, W; Gao, F; Gao, J; Gao, L; Gatchalian, E; Gatmaitan, A; Gavran, L; Ghaffar, A; Ghuman, D; Gilani, H; Glen, L; Goh, B; Goingco, R; Gomez, H; Gomez, O; Gondal, M; Gong, M; Gopalkrishna, N; Guan, S; Guanzon, V; Gundran, N; Guo, Y; Guo, J; Guo, J; Guo, Y; Gupta, A; Gupta, M; Gupta, P; Gupta, S; Gupta, S; Gustiawan, D; Gutierrez, M; Guz, L; Ha, S; Hafeez, A; Haleem, A; Ham, S; Hameed, A; Han, J; Han, J; Han, K; Hartono, T; Hassan Khan, M; Hendrawan, D; Herawati, N; Hermanto, S; Hermawan, D; Hindradi, W; Hong, KS; Hong, T; Hong, Z; Hongshu, X; Hor, S; Ho Thi, O; Hu, F; Hu, X; Hua, W; Hua, R; Huang, C; Huang, T; Huh, H; Hussain, S; Hussain, S; Hwang, H; Ibrahimpasic-Srna, K; Ijaz, M; Ikhsan, R; Indiyah, I; Iqbal, D; Iqbal, N; Irfan, M; Irfan, M; Iskandar, I; Ismail, S; Ivashina, E; Jameel, R; Jain, S; Jain, S; Jain, S; Jain, V; Jardeleza, R; Jayabal, A; Jeganathan Puloganathan, H; Jeon, J; Jeong, T; Jeremic, L; Ji, R; Jian, X; Jiandao, Y; Jie, H; Ju, Q; Jung, M; Jung, Y; Kahloon, E; Kang Hanwook; Kang, H; Kartikawati, N; Kasnawi, K; Katarzhanskaya, T; Katarzhanskiy, V; Kathiresan Pillai, N; Kayalvizhi, S; Khalid, A; Khan, A; Khan, D; Khan, D; Khan, S; Kheng, K; Khoo, Y; Ki, S; Kim, D; Kim, G; Kim, H; Kim, I; Kim, J; Kim, J; Kim, J; Kim, JM; Kim, K; Kim, K; Kim, M; Kim, P; Kim, S; Kim, S; Kim, S; Kim, S; Kim, T; Kim, T; Kim, Y; Kim, Y; Kim, Y; Kim, Y; Kim, Y; Kim, Y; Kiranmayi, P; Kiranmayi, P; Ko, S; Koh, C; Kolenda, D; Kothari, l; Korpusova, I; Kow, G; Krasilnikova, T; Kravchak, A; Krishan, V; Krishna, A; Kujundzic-Vatrenjak, F; Kumar, A; Kustagi, AL; Kuswanto, I; Kwan, G; Kwak, H; Kwok, S; Kwon, J; Ku, J; Kudinov, V; Kumar, P; Kung Lim, S; Kustova, N; Kuzmina, L; Lal Khatri, A; Latha, RM; Lana, W; Lasmaria, R; Lazatin, T; Lee, BI; Lee, H; Lee, J; Lee, J; Lee, J; Lee, K; Lee, K; Lee, S; Lee, S; Lee, S; Lee, S; Lee, S; Lee, S; Lee, W; Lee, Y; Lee, Y; Legaspi, M; Lei, X; Lestantyo, D; Li, D; Li, G; Li, J; Li, K; Li, L; Li, R; Li, W; Li, X; Li, Y; Li, Y; Liang, W; Liang, B; Liang, G; Liang, Z; Liannawati, L; Lin, X; Lin, X; Ling, H; Ling, W; Lim, C; Lim, S; Lim, W; Lipnicevic, S; Lismayani, I; Liu, C; Liu, H; Liu, H; Liu, J; Liu, J; Liu, S; Liu, S; Liu, W; Liu, X; Liu, Y; Lohano, V; Lontoc, M; Lou, X; Lu, J; Lucas-Linga, R; Lumbun, N; Lusida, J; Luo, Y; Lv, X; Lyu, S; Ma, L; Madni, A; Magaling, C; Magtolis, L; Mahfooz Khan, I; Mahfudz, F; Mahmud, R; Majeed, L; Mak, W; Makhrova, O; Malaki, L; Malik, Z; Mališ, S; Mamaril, R; Mao, L; Marbibi, A; Maria Victoria, D; Mariani, M; Martinez, E; Martinus, S; Markovic, R; Masood, D; Meenakshi, S; Megherbi, M; Mei Wulan, M; Membrebe, M; Memon, A; Memon, Q; Miao, G; Miklukova, V; Mimoun, L; Min, Z; Minina, I; Mishra, A; Mirasol, R; Miyan, Z; Mokhtari Mehni, R; Monalisa, M; Mondal, S; Moon J; Moon S; Mosbah Boulenmour, S; Muhammad, A; Mugiarti, S; Mukherjee, S; Mukti, S; Muljati, S; Mulyono, D; Munir, A; Mutha, A; Mushtaq, A; Music-Palalic, I; Mustafic, D; Nam Y; Nam Ko, S; Naeem, A; Ng, K; Ngan, S; Ngo Thanh, N; Nguyen Thi Bich, V; Nguyen Thi Ngoc, H; Nguyen Thu, H; Nguyen Thuy, T; Niu, X; Nidagundi, K; Nizami, U; Nicodemus, J; Oh, C; Oh, J; Oh, N; Oh, T; Onga, O; Orakzai, N; Orba Busro, V; Pan, X; Pande, T; Pandian, M; Panelo, A; Pang, Z; Panphilova, D; Paredes, F; Parikh, K; Parikh, P; Park, H; Park, J; Park, J; Park, J; Park, J; Park, K; Park, S; Pascasio, B; Patange, S; Patel, HS; Patel, K; Pathan, A; Peng, I; Peregrino, E; Pham Thuy, H; Philip, E; Ponce, R; Prabowo, P; Prajitno, E; Prasad, B; Prnjavorac, D; Purnomo, R; Purwanti, P; Purwono, R; Puspitarani, D; Puspitawati, I; Qiaomin, W; Qin, X; Qing, D; Qinyong, Y; Radic, B; Rafiq, K; Ragavan, D; Rahayu, W; Rane, N; Rasool, G; Rasool, G; Rasool, T; Rathi, K; Ravishankar, AG; Raza, A; Rehman, F; Remaldora, M; Ringkananont, U; Rizal, W; Rozajac, J; Roziati, M; Ryu, O; Saeed, T; Sagoro, D; Sakrani, A; Salihovic, H; Sangguna, A; Sansanayudh, N; Santoso, A; Santos, F; Sapang, B; Savenkova, N; Selimovic, S; Selvakumar, P; Selvapandian, P; Sembiring, D; Semilla, N; Seng, S; Sentosa, S; Septia, D; Serebrennikova, E; Setijo, H; Setiogung, B; Shah, JD; Shah, K; Shamkaeva, R; Shan, Y; Shani, D; Shao, Z; Sheikh, D; Shi, Y; Shin, D; Shin, S; Shoaib Qureshi, S; Shmeleva, E; Shuai, W; Shubing, l; Shujuan, Y; Shun, X; Shun, Y; Shunkai, Z; Shurigin, V; Si, W; Simatupang, E; Singh, H; Singh, NP; Singh, P; Singh, P; Singh, R; Singh, R; Sinorita, H; Sinuraya, G; Sohail, D; Sok, B; Som, L; Son, KH; Song, S; Soon, C; Sor, D; Sreetharanathan, R; Srimanarayana, K; Srivastav, H; Srivastav, S; Srivastav, SB; Stevanovic, R; Storozhok, M; Sudjono, S; Suh, J; Suljic, S; Sulistiyanto, H; Sum, S; Supinto, S; Suprayitno, S; Sura-amornkul, S; Surya, A; Suryabudhi, E; Suryaminah, S; Susilo W, H; Sutanto, I; Suwarni, I; Syam, P; Synanyan, T; Tabangcura, P; Tack, Y; Tahir, S; Talla, C; Tama, K; Tan, C; Tan, G; Tan, G; Tan, L; Tan, M; Tanchoco, E; Tanumihardja, B; Tanweer, A; Tarasova, V; Tarueno, A; Tarwani, H; Tat, C; Teoh, K; Teow, R; Terzic, M; The, P; Thomas, J; Tiu, F; Tkachenko, E; Tobing, D; Tokic, F; Tolentino, M; Tong, P; Tongson, R; Toor, H; Toreja,T; Touch, K; Tsang, C; Tsang, K; Tsvetkovskaya, T; Tran Thi Thanh, H; Trivedi, S; Tulod, E; Tuo, H; Turmukhambetova, B; Ud-din, M; Ugalino, C; Un, H; Ur-Rehman, N; Usman; Vagapova, G; Vaichulis, O; Vaja, C; Valdez, G; Valdez, M; Valerio, J; Vanja, S; Verdillo, L; Verma, K; Villar, R; Vilenskaya, G; Vithalani, M; Vladimirova, T; Vorobyev, S; Wang, C; Wang, D; Wang, D; Wang, F; Wang, F; Wang, H; Wang, l; Wang, N; Wang, W; Wang, X; Wang, X; Wang, Y; Wangi, H; Wanharni, W; Wardhana, A; Wardhani, T; Warraich, D; Wei, A; Wei, F; Wei, H; Weifang, H; Weiguo, H; Weijia, Z; Wenyan, W; Wenyong, S; Wenzhao, W; Widayati, W; Widjayani, A; Widjojo, S; Widura, W; Widya Saputra, L; Winantea, W; Wiyono, H; Won, S; Wong, A; Wong, C; Wong, C; Wong, K; Wong, T; Wong, T; Wongso, M; Wu, J; Wu, S; Wu, X; Xia, J; Xiao, W; Xiaowen, C; Xiaoyan, C; Xiaoying, Z; Xin, N; Xiushi, N; Xu, Z; Xu, Z; Xu, A; Xu, N; Xu, Y; Xuefeng, D; Y. Edi, S; Yadav, A; Yahya, N; Yakhou, M; Yang, G; Yang, N; Yang, S; Yao, C; Yazdani Khan, K; Ye, l; Ye, Y; Yew, F; Yii, K; Yimin, S; Yong, l; Yong, Y; Yousuf, J; Yu, J; Yuan, H; Yueping, W; Yu-Gan, S; Yun, K; Yun, S; Yun, T; Yuneva, G; Yuwu, Z; Yuyou, Z; Zhai, G; Zhang, C; Zhang, D; Zhang, G; Zhang, G; Zhang, H; Zhang, J; Zhang, N; Zhang, R; Zhang, W; Zhang, X; Zhang, X; Zhang, X; Zhang, X; Zhang, Y; Zhang, Z; Zhao, H; Zhao, H; Zhao, Y; Zhao, Z; Zhimin, W; Zhong, C; Zhou, J; Zhou, l; Zhou, X; Zhou, Y; Zhu, Y; Zhuhua, Z; Zainab, Z; Zaffar, J; Zahid, N; Zaidi, S; Zia, F.
Rights and permissions
About this article
Cite this article
Zhang, W., Kim, D., Philip, E. et al. A Multinational, Observational Study to Investigate the Efficacy, Safety and Tolerability of Acarbose as Add-On or Monotherapy in a Range of Patients: The GlucoVIP Study. Clin Drug Investig 33, 263–274 (2013). https://doi.org/10.1007/s40261-013-0063-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0063-3