Abstract
Relatively little is known about the influence of extreme body weight on the pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety of drugs used in many disease states. While direct oral anticoagulants (DOACs) have an advantage over warfarin in that they do not require routine drug monitoring, some may regard this convenience as less compelling in obese patients. Some consensus guidelines discourage using DOACs in patients weighing > 120 kg or with a body mass index > 35–40 kg/m2, given a sparsity of available data in this population and the concern that fixed dosing in obese patients might lead to decreased drug exposure and lower efficacy. Per the prescribing information, apixaban does not require dose adjustment in patients weighing above a certain threshold (e.g., ≥ 120 kg). Data from healthy volunteers and patients with nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) have shown that increased body weight has a modest effect on apixaban’s PK. However, the paucity of exposure data in individuals > 120 kg and the lack of guideline consensus on DOAC use in obese patients continue to raise concerns about potential decreased drug exposure at extreme weight. This article is the first to comprehensively review the available PK data in obese individuals without NVAF or VTE, and PK, PD, efficacy, effectiveness, and safety data for apixaban in obese patients with either NVAF or VTE, including subgroup analyses across randomized controlled trials and observational (real-world) studies. These data suggest that obesity does not substantially influence the efficacy, effectiveness, or safety of apixaban in these patients.
Trial Registration ARISTOTLE: NCT00412984; AVERROES: NCT00496769; AMPLIFY: NCT00643201; AMPLIFY-EXT: NCT00633893; ADVANCE-1: NCT00371683; ADVANCE-2: NCT00452530; ADVANCE-3: NCT00423319
Video abstract
Apixaban Use in Obese Patients: A Review of the Pharmacokinetic, Interventional, and Observational Study Data (MP4 161.22 MB)
Similar content being viewed by others
There is a lack of consensus regarding direct oral anticoagulant dosing for the management of nonvalvular atrial fibrillation (NVAF) and venous thromboembolism (VTE) in obese patients, particularly morbidly obese patients, given the relative scarcity of randomized clinical trial data in this population. Guidelines generally discourage their use in patients with a body mass index > 35–40 kg/m2 or weight > 120 kg. |
Exposure to apixaban, as indicated by the area under the plasma concentration–time curve, is modestly reduced in obese patients, but not to a degree that warrants dose adjustment. |
Accumulated data from randomized clinical trials and observational (real-world) studies suggest that increased body weight has no impact on apixaban’s efficacy or effectiveness and safety in patients with NVAF or VTE. |
1 Introduction
Relatively little is known about the influence of extreme body weight on the clinical pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety of drugs used in many disease states, including the direct oral anticoagulants (DOACs) in prothrombotic disorders such as nonvalvular atrial fibrillation (NVAF) and venous thromboembolism (VTE).
Overweight and obesity are frequently categorized according to the following body mass index (BMI) categories [1,2,3]:
-
Overweight: BMI 25 to < 30 kg/m2.
-
Class 1 obesity: BMI of 30 to < 35 kg/m2.
-
Class 2 obesity: BMI of 35 to < 40 kg/m2.
-
Class 3 obesity: BMI of ≥ 40 kg/m2 (also categorized as “severe” or “morbid” obesity).
The European Society of Cardiology further defines class 4 or “super-obesity” as BMI ≥ 50 kg/m2, and class 5 or “super-super or extreme obesity” as BMI ≥ 60 kg/m2 [4].
“Morbid” obesity is somewhat variously defined according to weight, ideal body weight, BMI alone, waist circumference, or weight-exacerbated comorbid conditions. For example, according to the University of Rochester, “An individual is considered morbidly obese if he or she is 100 pounds over his/her ideal body weight, has a BMI of ≥ 40 kg/m2, or ≥ 35 kg/m2 and experiencing obesity-related health conditions, such as high blood pressure or diabetes” [5]. Diagnostic codes for severe obesity with multiple, largely BMI-based synonyms are available in the International Classification of Diseases 9 and 10 coding systems [6].
The prevalence of both obesity and morbid obesity has been increasing sharply over the past decades [7, 8]. Data from the US Centers for Disease Control show that from 1999 through 2018, the prevalence of obesity (BMI ≥ 30 kg/m2) and severe obesity (BMI ≥ 40 kg/m2) in adults (≥ 20 years) increased from 30.5 to 42.4% and from 4.7 to 9.2%, respectively [7]. In 2014, the average adult body weight in the United States was 88.8 kg (5th and 95th percentiles, 62.0 and 124.9 kg) for males and 76.4 kg (5th and 95th percentiles, 50.1 and 116.5 kg) for females [9]. It is estimated that 38% of the world’s adult population will be overweight and another 20% will be obese by 2030 [10].
Although some studies have suggested that obesity may be associated with paradoxically improved survival and thrombotic outcomes in patients with NVAF [11,12,13,14,15], obesity is nevertheless clearly recognized as a biologically plausible independent risk factor for developing NVAF [11,12,13]. In a longitudinal cohort study of 67,278 patients, half of whom were obese, after over 8 years of follow-up, obesity was strongly associated with a new diagnosis of NVAF after controlling for differences in age, gender, hypertension, and diabetes [16]. In addition, the contribution of obesity as an independent risk factor for stroke in patients with NVAF remains unclear [17]. Two observational studies have shown that there was an increased rate of stroke in obese patients with NVAF relative to those with normal body weight [18, 19]. A separate observational study showed that there was an inverse relationship between BMI and stroke severity in patients with acute ischemic stroke and NVAF, with lower stroke severity in patients with higher body weights [20].
As with NVAF [21], some studies have also suggested an obesity–mortality paradox also exists in patients with VTE. Nevertheless, obesity is clearly associated with first VTE, is less clearly associated with recurrent VTE, and is recognized to interact with other risk factors for VTE [22,23,24]. Obesity is a risk factor in certain VTE risk assessment scores, including HERDOO2 for recurrent unprovoked VTE in women [23, 25], the Padua Prediction Score for hospitalized medical patients [26], and the Khorana risk stratification score for cancer-associated VTE [27]. By contrast, however, obesity is not a recognized risk factor in other risk stratification models such as the Vienna Risk Model [22] and the DASH Prediction Score [28], both for recurrent unprovoked VTE.
Consensus guidelines for anticoagulant therapy in NVAF or VTE have either discussed obesity obliquely or discouraged DOAC use in patients weighing > 120 kg, or with a BMI > 35–40 kg/m2. However, the recent 2021 International Society on Thrombosis and Haemostasis (ISTH) guidelines suggest that standard doses of apixaban or rivaroxaban, along with vitamin K antagonists (VKAs), weight-based low molecular weight heparin (LMWH), and fondaparinux, are among appropriate anticoagulation options regardless of high BMI and weight for the treatment of VTE and VTE prophylaxis after hip or knee replacement surgery [29]. Additionally, and in contrast to previous recommendations (2016) [30], the ISTH guidelines no longer suggest regularly monitoring peak or trough drug-specific DOAC levels because there are insufficient data to influence management decisions. The 2016 AC Forum Guidance on DOAC use for VTE treatment suggests avoiding DOACs in patients with VTE at extremes of body weight/BMI (e.g., weight < 50 kg or > 120 kg, or BMI ≥ 35 kg/m2) pending availability of more data [31].
Other guidelines on VTE management do not provide clear guidance on use of DOACs in obese patients. For example, the 2021 update of the CHEST Guideline and Expert Panel Report on antithrombotic therapy for VTE disease states that certain clinical situations, such as extremes of weight, may favor use of VKAs over DOACs [32]. The American Society of Hematology 2021 guidelines for management of VTE in patients with cancer note that additional information is needed on the dosing of anticoagulation for obese patients [33]. For NVAF, the 2021 European Heart Rhythm Association practical guide on the use of DOACs in NVAF recommends using DOACs with caution or consideration of VKA use in patients with a BMI ≥ 40 kg/m2 or weight > 120 kg [34]. The 2019 focused update by the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) does not make a specific recommendation, but suggests DOAC level measurements might be indicated for evaluation of drug absorption in patients > 120 kg or with a BMI > 35 kg/m2 [35]. The recommendation to routinely check DOAC exposure levels in high body weight patients has recently been challenged [36].
Regardless of consensus guidelines, DOACs continue to be used in the obese patient population. For example, a recently published study in an urban university setting reveals that DOACs were prescribed to patients with morbid obesity (weight > 120 kg or BMI > 40 kg/m2) [37]. Data from the GLORIA-AF registry show not only that obesity in general is associated with increased oral anticoagulant (OAC) prescription rates in NVAF, but also suggest that moderate and severe obesity tend to favor the use of DOACs over warfarin [38].
Apixaban is a selective inhibitor of activated coagulation factor Xa in the class of DOACs. Apixaban is approved for use in the United States to reduce the risk of stroke and systemic embolism (SE) in adult patients with NVAF, for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), for the reduction in the risk of recurrent DVT and PE following initial therapy, and for the prophylaxis of DVT, which may lead to PE, in patients who have undergone hip or knee replacement surgery [39].
According to US product labeling of apixaban based on PK studies and subgroup analyses of the pivotal randomized controlled trials (RCTs), no dose adjustment is necessary for any single patient characteristic, including body weight alone [40]. The recommended dosage should be reduced from 5 to 2.5 mg orally twice daily (bid) only in patients with NVAF who have two or more of the following characteristics: age ≥ 80 years, body weight ≤ 60 kg, or serum creatinine ≥ 1.5 mg/dL (commonly referred to as the “ABC” [age, body weight, creatinine] criteria) [39]. According to the European Summary of Product Characteristics, in patients with severe renal impairment (creatinine clearance 15–29 mL/min), the following additional recommendations apply: for the treatment of DVT, treatment of PE, and prevention of recurrent DVT and PE, apixaban is to be used with caution; for the prevention of stroke and SE in patients with NVAF, patients should receive the lower dose of apixaban 2.5 mg bid [41].
Apixaban, like other DOACs, was developed with the advantage over warfarin that therapeutic drug monitoring is not required for use. However, clinical decisions (e.g., overdose and emergency surgery) may be better informed by knowledge of apixaban exposure. Although the chromogenic assay used to support apixaban clinical development reported anti-factor Xa activity in LMWH units, current assays are available using an apixaban-specific calibrator for which anti-factor Xa activity values are reported in apixaban concentration units, enabling a timely evaluation of apixaban exposure [42]. Predicted steady-state apixaban and anti-factor Xa activity levels are available for each indication and at each dose level. To the best of our knowledge, the HemosIL® Liquid Anti-Xa assay (Instrumentation Laboratory, Bedford, MA, USA) is the only apixaban assay currently approved by the US Food and Drug Administration for clinical practice [43].
Exposure–response analyses of apixaban in NVAF and VTE studies have shown that there is no defined therapeutic exposure range or discernible threshold of apixaban concentration that would predict efficacy outcomes for a given individual [42]. For this reason, except in the above unique situations, measurement of apixaban concentration to guide dose selection is not recommended.
Concerns regarding dosing of DOACs in obese patients persist widely in the medical community, due to a perceived risk of decreased efficacy resulting from hypothetical sub-therapeutic exposure. To provide a comprehensive resource on use of apixaban in obese patients, we summarized all available PK and clinical outcomes data for apixaban across weight categories, from both pivotal RCTs and observational studies. This review represents the most complete summary of apixaban PK, PD and clinical outcome data to date, collectively demonstrating that the safety and efficacy of apixaban remain consistent across body weights using standard prescribing information (PI) dosing guidance.
2 Objective
The following review is intended as a comprehensive summary of available data from published literature of apixaban’s PK and PD, clinical trial efficacy, real-world effectiveness, and safety in obesity.
3 Methodology of the Literature Search and Review
We primarily searched available PK and clinical data on apixaban use in obese patients with NVAF or VTE, those receiving VTE prophylaxis, or healthy volunteers. The majority of the studies of interest were expected to include comparisons of apixaban with warfarin or other DOACs. Of note, the registration RCTs compared apixaban to warfarin, and there are no large-scale RCTs comparing DOACs head-to-head. However, there are many retrospective studies making multiple pairwise comparisons between and among DOACs and warfarin. For this review, we have included retrospective observational studies and meta-analyses of RCTs that compare apixaban to warfarin, or in the case of multi-DOAC analyses, those that provide data specific to apixaban. This review does not include analyses of pooled DOAC groups where estimates are shown only for the comparisons of combined DOACs with warfarin.
We interrogated PubMed via query on i2e, a text mining application that adopts natural language processing (NLP) [44]. We searched specifically for publications through March 2021 that met each of three criteria, found in either the publication’s title or abstract. These include the authors mentioning (1) apixaban or other medicine described as a non–vitamin K antagonist oral anticoagulant (NOAC)/DOAC; (2) some form of obesity; and (3) an indication approved in the PI for apixaban. To catch more abstracts, we queried for synonyms of key terms noted in Table 1, after deriving synonyms for apixaban from the National Cancer Institute's thesaurus and synonyms for indications from Linguamatics’ nlm.plus ontology.
We sought to ensure that online abstracts meeting these criteria were specific to obesity and that they met the criteria in a clinically relevant context. To that end, our NLP strategy prioritized publications where the abstract specified a sample size for obese patients; where “obesity” and its synonyms were used in phrases that also included the terms “patients,” “females,” “males,” “women,” “men,” “subjects,” “cohorts,” “populations,” and “individuals”; and where the relationship between the indication and the pharmacotherapy was syntactically explicit. For the latter, we specifically constrained the query to search for phrases which simultaneously include the drug, the indication, and one of the following words: “treatment,” “use,” “reduce,” “risk,” “prevent,” “decrease,” “lower,” “improve,” “prophylaxis,” “recommend,” “approve,” “prefer,” “for,” and “with.” We also added to this terminology a list of Linguamatics’ Improve Disease verbs [44].
To exclude any possible irrelevant results, one of the authors (AQ) screened the publication meta-data. Three authors (RD, JO, and MJ) then reviewed the remaining publications to confirm that they met our criteria for review.
For each study, the following basic characteristics were considered: publication year, study type, sample size, type and dosage of DOACs, BMI or body weight categories, outcomes, and follow-up time. Studies were considered eligible for reference if they (1) were designed as subgroups of phase III RCTs, post hoc analyses of RCTs, or observational cohorts (prospective or retrospective), and (2) reported the impact of BMI or body weight on outcomes in patients with NVAF or VTE taking apixaban. We excluded review articles, commentaries, editorials or letters, case studies, conference abstracts, and preclinical studies.
A number of apixaban trials included substantial numbers of patients with obesity but have not reported outcomes based on higher weights, and are therefore not included in this review. These include the ADAM, AMPLIFY-EXT, AVERT, AVERROES, AUGUSTUS, and CARAVAGGIO trials [45,46,47,48,49,50].
4 The Effects of Obesity on the Clinical PK of Apixaban
A detailed overview of apixaban’s PK profile has been published [42]. Apixaban is rapidly absorbed without a clinically meaningful impact of food on the bioavailability: absolute oral bioavailability is approximately 50%. Apixaban exposure increases dose-proportionally for oral doses up to 10 mg. Apixaban has a half-life of approximately 12 h. Elimination occurs via multiple pathways including metabolism, biliary excretion, and direct intestinal excretion, with approximately 27% of total apixaban clearance occurring via renal excretion. The PK of apixaban are consistent across a broad range of patients, and apixaban has limited clinically relevant interactions with most commonly prescribed medications, allowing for fixed dosages without the need for therapeutic drug monitoring.
Commonly referred to as the allometric concept [51], body weight could be an important covariate to explain variability of PK parameters such as clearance or volume of distribution. In particular, obesity could alter the disposition of a drug and potentially result in changes in its benefit–risk profile [52, 53]. As obese patients have a larger body mass with a smaller proportion of lean body mass compared with non-obese patients, this could alter the distribution of drugs by impacting the volume of distribution. The volume of distribution for apixaban is approximately 21 L [42], similar to the estimated volume of extracellular fluid [54]. This suggests that apixaban is mainly distributed into extracellular fluid, with limited intracellular distribution. Here, we review the impact of body weight and obesity on the PK of apixaban.
4.1 Impact of Body Weight on PK and PD of Apixaban in Healthy Volunteers
An open-label, single-dose, parallel-group study investigated the effect of extremes of body weight on the PK, PD, safety, and tolerability of a single 10-mg oral dose of apixaban in healthy individuals aged 18–45 years [53]. The study population was categorized by weight categories as low (≤ 50 kg, n = 18), reference (65–85 kg, n = 16), and high (≥ 120 kg, n = 19). Individuals in the high body weight group were also required to have a BMI ≥ 30 kg/m2, while individuals in the low and reference body weight categories were required to have a BMI ≤ 30 kg/m2. Mean (standard deviation) BMI in the three groups was 18.8 (2), 26.3 (2), and 42.6 (6), respectively. Exposure to apixaban, as represented by the area under the concentration–time curve (AUC) to infinity was 23% lower in the high weight group when compared with the reference weight group (Table 2). Considering the lack of a trend between renal clearance (CLR) and body weight, the observed modest increase in apparent clearance of apixaban (CLT/F) may result from increased non-renal elimination pathways of apixaban. Body weight does not appear to influence the relationship between apixaban and anti-factor Xa activity [53], a primary PD effect [55].
4.2 Pharmacokinetics of Apixaban in Patients with NVAF or VTE from Subgroup Analyses of RCTs
In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial [56], 18,201 patients with NVAF and at least one additional risk factor for stroke were randomized 1:1 in a double-blind, double-dummy fashion, to apixaban 5 mg bid (or 2.5 mg bid, for patients meeting at least two of the “ABC” criteria) or to warfarin titrated to an international normalized ratio (INR) between 2 and 3. Further details of the study are discussed in Sect. 5.1.
A population PK analysis, conducted to characterize apixaban exposure in 2932 patients with NVAF enrolled in the ARISTOTLE trial who received apixaban (5 mg bid, n = 2804; 2.5 mg bid, n = 128), has been published [57]. Figure 1 shows the relationship between steady-state AUC and body weight in 2804 patients treated with apixaban 5 mg bid in the ARISTOTLE trial. In a single linear regression analysis, body weight accounted for 21% of the variance in AUC.
In the Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial, apixaban (10 mg bid for 7 days followed by 5 mg bid) was non-inferior to enoxaparin (1 mg/kg bid for 7 days) taken concomitantly with warfarin (target INR 2.0–3.0) over 6 months for the outcome of VTE or VTE-related death, with significantly less major bleeding (MB) [46]. Further details of the trial are discussed in Section 5.2.
A population PK analysis was conducted to characterize apixaban exposure in 281 patients in AMPLIFY [58]. A recently published post hoc analysis of AMPLIFY patients from the same population PK analysis result showed a similar inverse relationship between steady-state AUC and body weight to that in NVAF (Fig. 2) [59]. In summary, the population PK analysis of apixaban in patients undergoing VTE treatment was used to predict steady-state daily (0–24 h) exposure for each patient based on the empirical Bayes prediction of their oral clearance value and total daily dosage of apixaban. The results showed that there was a modest (< 30%) decrease in the median predicted exposure with increasing body weight, which was considered not clinically meaningful.

Reproduced from Cohen AT, Pan S, Byon W, et al. Efficacy, Safety, and Exposure of Apixaban in Patients with High Body Weight or Obesity and Venous Thromboembolism: Insights from AMPLIFY. Adv Ther. 2021;38:3003–18 [59], https://link.springer.com/article/10.1007/s12325-021-01716-8, under Creative Commons license 4.0 (CC BY-NC-4.0)
Predicted steady-state daily AUC by baseline body weight category in participants in the AMPLIFY trial [59]. Boxes = 25th to 75th percentiles; whiskers = 5th to 95th percentiles; numbers inside boxes = median values; circles = individual predicted values. AUC area under the concentration–time curve.
4.3 Other Studies of Apixaban PK in Patients with NVAF or VTE Weighing > 120 kg
Martin et al. conducted a prospective observational study of DOAC PK and PD in 100 patients with NVAF or VTE weighing > 120 kg (88% with BMI > 40 kg/m2) receiving apixaban 5 mg bid (n = 11 peak and n = 11 trough in the NVAF cohort; n = 8 peak and n = 4 trough in the VTE cohort) or rivaroxaban 20 mg once daily (QD) (n = 22 peak and n = 16 trough in the NVAF cohort; n = 36 peak and n = 5 trough in the VTE cohort), at two centers in the United Kingdom [36]. They found no significant linear relationships between peak or trough DOAC concentrations and either body weight or BMI. These patients generally achieved anti-factor Xa concentrations in the expected “therapeutic” ranges for each condition, as defined by the International Council for Standardization in Haematology [60]. Ninety-five percent of trough concentrations were within the expected range, suggesting no under-dosing in the majority of these patients.
In 46 patients receiving apixaban for NVAF or VTE, Wasam and colleagues reported that increases in apixaban plasma concentrations and anti-factor Xa activity, between 2 and 4 h after dosing, were blunted in those weighing > 120 kg (n = 23) versus those weighing < 120 kg [61].
5 Efficacy and Safety of Apixaban in Patients with Obesity, According to Disease State
5.1 Introduction
Table 3 summarizes baseline body weight distributions in apixaban’s registration trials for NVAF and VTE. Individual trial data are discussed below. The ADVANCE 1, 2, and 3 knee and hip replacement trials excluded individuals weighing > 136 kg or having BMI ≥ 35 kg/m2 unless the investigator could assure that bilateral venography was technically feasible (data on file). The remaining listed trials did not exclude patients based on body weight.
Several systematic reviews and/or meta-analyses of DOAC use in obese patients with NVAF [13, 62,63,64,65], VTE [66,67,68], or both [62, 69] have been published. Detailed discussion of each is outside the scope of this article, but in general the analyses suggest that in obese and non-obese patient populations, there were no significant differences between apixaban and warfarin treatment for the outcomes of stroke, SE, recurrent VTE, or MB associated with apixaban versus warfarin use in obese versus non-obese patient populations.
5.2 Patients with NVAF
In the ARISTOTLE trial, apixaban was compared with warfarin in patients with NVAF for the prevention of stroke or SE (primary efficacy outcome) and ISTH MB (primary safety outcome) in patients with NVAF [56]. The secondary efficacy endpoints included all-cause death, myocardial infarction, and individual components of the primary outcome (stroke, ischemic or uncertain stroke, and hemorrhagic stroke). Secondary safety endpoints were major or clinically relevant non-major (CRNM) bleeding, intracranial hemorrhage, gastrointestinal bleeding, or any bleeding. In two post hoc analyses of ARISTOTLE, the primary and key secondary outcomes were analyzed in subsets of patients with available baseline (1) body weight or (2) BMI [12, 70].
The body weight analysis [70] categorized 18,139 patients into strata for low weight (≤ 60 kg; 11%), mid-range weight (> 60 to 120 kg; 84%) or high weight (> 120 kg; 5%). In an additional sensitivity analysis, patients with high body weight were further categorized as either weighing 121–140 kg (4%) or > 140 kg (1%). Compared with patients treated with warfarin, those treated with apixaban had lower absolute rates of stroke or SE across all weight categories (Fig. 3). Additionally, when treated as a continuous variable, there was an interaction between weight and treatment for the outcome of hemorrhagic stroke (P = 0.0418) ≤ 60 kg and 61–120 kg. There were four hemorrhagic strokes in patients weighing > 120 kg, all of whom were taking warfarin.

Reproduced with permission from: Hohnsloser SH et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients With Atrial Fibrillation and Extremes in Body Weight. Circulation. 2019;139(20):2292–2300 [70]; https://www.ahajournals.org/journal/circ
Efficacy and safety outcomes for apixaban vs warfarin, with weight as a continuous variable, in patients with NVAF in the ARISTOTLE trial [70]. A Stroke or SE; B all-cause death; C myocardial infarction; D ISTH major bleeding. ISTH International Society on Thrombosis and Haemostasis, NVAF nonvalvular atrial fibrillation, SE systemic embolism.
This body weight analysis also demonstrated significant interactions between body weight and treatment for MB and MB or CRNM bleeding. In neither case did this reflect comparatively lower bleeding rates with apixaban versus warfarin in patients > 120 kg (as might be expected in theoretically under-dosed patients).
In addition to comparing warfarin and apixaban across various categorical weight strata, the body weight analysis treated weight as a continuous variable, as illustrated in Figs. 3 and 4. Fig. 3 illustrates the efficacy (stroke or SE, myocardial infarction and all-cause mortality) and safety (ISTH MB) of apixaban compared with warfarin across the weight continuum, up to approximately 145 kg. Fig. 4 illustrates the rates of stroke or SE and ISTH MB across discrete weight categories (≤ 60, 61–120, and > 120 kg).
Body weight categories vs relative risk of A stroke or SE and B ISTH MB in patients with NVAF in the ARISTOTLE trial [70]. ISTH International Society on Thrombosis and Haemostasis, MB major bleeding, NVAF nonvalvular atrial fibrillation, SE systemic embolism
In patients who weighed > 140 kg, three stroke or SE events (apixaban, n = 2; warfarin, n = 1) and five MB events occurred (warfarin, n = 5). It should be noted that due to the small number of events, this analysis was not powered to detect a treatment interaction between apixaban- and warfarin-treated patients and body weight group.
The separate BMI analysis [12] included 17,913 patients with a BMI ≥ 18.5 kg/m2 and categorized them as normal (BMI 18.5 to < 25 kg/m2), overweight (25 to < 30 kg/m2), and obese (≥ 30 kg/m2). Patients from the highest BMI group were further categorized into BMIs of either ≥ 30 to < 35 kg/m2, 35 to < 40 kg/m2, or ≥ 40 kg/m2 for additional sensitivity analysis. The principal analysis compared efficacy and safety outcomes in overweight patients relative to those with a normal BMI, as well as in obese patients relative to normal weight individuals, for each treatment. In multivariable analyses, overweight and obese patients were significantly associated with a lower risk of all-cause mortality and a lower risk of the composite endpoint of stroke or SE, myocardial infarction, and all-cause mortality. These findings were described by the authors as the “obesity paradox.”
When evaluated with BMI as a continuous variable, apixaban, relative to warfarin, was associated with a lower risk of stroke or SE, all-cause mortality, the composite endpoint, and MB across the range of BMI categories, with no significant interaction between the treatment effect and higher BMI. In patients with a BMI < 30 kg/m2, a significant interaction between BMI and treatment was only observed for MB (P = 0.01), reflecting a greater treatment effect and favoring apixaban.
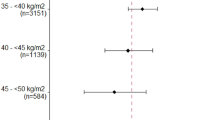
Although not powered to detect an interaction between treatment and BMI category, the sensitivity analysis (BMI ≥ 30 to < 35 kg/m2, 35 to < 40 kg/m2, and ≥ 40 kg/m2) showed that the association between BMI categories and the efficacy and safety endpoints remained unchanged.
These studies share the limitations of post hoc subgroup analyses of RCTs in that they are underpowered to detect treatment interactions. Both used baseline weight only; therefore, the impact of any changes in weight over time could not be assessed.
5.3 Patients with VTE
The AMPLIFY trial is described in Sect. 4.3 [46]. A post hoc analysis of the AMPLIFY trial was conducted to evaluate the efficacy and safety of, and exposure to, apixaban for the treatment of VTE in patients across body weight categories, including patients with a body weight ≥ 120 kg or BMI > 40 kg/m2 [59]. Of the 5395 patients who were randomized and included in the safety population of AMPLIFY, 5384 patients had body weight recorded. Of these, 476 patients weighed ≤ 60 kg, 3868 patients weighed > 60 to < 100 kg, 750 patients weighed ≥ 100 to < 120 kg, and 290 patients weighed ≥ 120 kg. Across different body weight (Fig. 5) or BMI categories, including those who weighed ≥ 120 kg, compared with enoxaparin/warfarin, apixaban had similar rates of VTE or VTE-related death and lower rates of bleeding (MB and major or CRNM bleeding). The safety and efficacy of apixaban in patients with extremes of body weight were consistent with the main results of the AMPLIFY trial; however, due to the low numbers of patients with a body weight ≥ 120 kg or BMI > 40 kg/m2, this analysis was underpowered to detect a difference between treatment arms.

Reproduced from Cohen AT, Pan S, Byon W, et al. Efficacy, Safety, and Exposure of Apixaban in Patients with High Body Weight or Obesity and Venous Thromboembolism: Insights from AMPLIFY. Adv Ther. 2021;38:3003–3018 [59], https://link.springer.com/article/10.1007/s12325-021-01716-8, under Creative Commons license 4.0 (CC BY-NC-4.0)
Recurrent VTE or VTE-related death and MB in 5384 participants in the AMPLIFY trial, during the treatment period, by baseline body weight category [59]. CI confidence interval, CRNM major or clinically relevant non-major, ISTH International Society on Thrombosis and Haemostasis, MB major bleeding, N number of participants, n number of events, NE not estimable, RR relative risk, VTE venous thromboembolism.
5.4 Patients at Risk of DVT After Hip or Knee Replacement Surgery
The ADVANCE-2 and ADVANCE-3 trials compared apixaban 2.5 mg bid with enoxaparin 40 mg QD subcutaneously for prevention of DVT, PE, or all-cause death in patients undergoing total knee (ADVANCE-2) or hip (ADVANCE-3) replacement [71, 72]. In ADVANCE-2, both regimens were continued for 10–14 days; in ADVANCE-3, the regimens were continued for 32–38 days.
A prespecified analysis, using pooled data from ADVANCE-2 and ADVANCE-3, assessed treatment differences across various subgroups, including BMI and body weight [73]. BMI subgroups were divided into < 25 kg/m2, 25–29 kg/m2, and ≥ 30 kg/m2. The prespecified outcome measure for efficacy in the subgroup analysis was major VTE, defined as the composite of adjudicated symptomatic or asymptomatic proximal DVT (popliteal, femoral, or iliac-vein thrombosis), non-fatal PE, and VTE-related death during the intended treatment period. The prespecified outcome measures for bleeding were MB and the composite of major and CRNM bleeding during the study treatment period. Of the 6767 patients with BMI data available who were evaluable for efficacy, 2388 (35%) had a BMI > 30 kg/m2. The efficacy of apixaban compared with warfarin was maintained in all three BMI subgroups (Pinteraction = 0.2273), with no difference in MB (Pinteraction = 0.5082) or the composite of MB and CRNM bleeding (Pinteraction = 0.3223), although the analysis was not powered to detect differences between treatments.
5.5 Bariatric Surgery
There are no published interventional studies on the safety or efficacy of apixaban in prophylactic dosages for prevention of DVT or PE in patients undergoing bariatric surgery, nor are there adequate studies of treatment doses of DOACs in the management of NVAF, VTE, or other indications in patients who have previously undergone these procedures [74]. Apixaban is not indicated for the prevention of DVT in patients undergoing bariatric surgery. A comprehensive review of the DOACs in bariatric surgery is included in the recent ISTH review of anticoagulant use for VTE in obesity [29].
6 Observational (Real-World) Studies
6.1 Introduction
Recently, several retrospective observational cohort studies have investigated the effectiveness and safety of apixaban in obese and morbidly obese patients with NVAF [75,76,77,78,79], VTE [80,81,82], or either NVAF or VTE [36, 83,84,85,86]. Results from these observational studies are largely confirmatory of the efficacy and safety data presented in the controlled clinical trials. No signals of reduced efficacy or safety are apparent. Many of these studies are limited by their small sample size, potential confounding, missing or misclassified data, potential for unmeasured bias, and lack of generalizability. Due to the observational nature of the claims database studies, outcome measures could only be based on claims codes. Body measurements such as weight are not available in the claims data. Modest sensitivity of the diagnosis codes used to identify obesity and severe obesity suggest that studies may fail to identify some of these patients [87].
6.2 NVAF
An analysis by Briasoulis et al. was conducted using cohorts of obese (≥ 120 kg) and morbidly obese (BMI > 40 kg/m2) patients with NVAF who were enrolled in the Veterans Health Administration system between 2012 and 2018 and who initiated apixaban, rivaroxaban, dabigatran, or warfarin [88]. At baseline, patients on apixaban had the highest rates of renal failure, previous stroke, and myocardial infarction compared to patients on other OACs. In obese patients (apixaban, n = 6052; rivaroxaban, n = 4309; dabigatran, n = 4233; warfarin, n = 13,417), compared with warfarin, all DOACs were associated with a lower risk of any hemorrhage, hemorrhagic stroke, and gastrointestinal bleeding while maintaining efficacy on ischemic stroke prevention. All-cause mortality was also lower with dabigatran and rivaroxaban than with apixaban or warfarin in these patients. In morbidly obese patients (apixaban, n = 3414; rivaroxaban, n = 2340; dabigatran, n = 2405; warfarin, n = 8267) all-cause mortality was also lower with dabigatran and rivaroxaban compared with apixaban and was lower with all three DOACs compared with warfarin. The authors note that differences in all-cause mortality among DOACs may represent heterogeneous populations and variable comorbidities not captured rather than differential effects on thromboembolic and bleeding risk.
To date, the ARISTOPHANES (Anticoagulants for Reduction In Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients; NCT03087487) obesity subgroup analysis is the largest retrospective observational study evaluating the risk of stroke or SE and MB among obese patients with NVAF who initiated OAC treatment [89]. This study used pooled data from the Centers for Medicare & Medicaid Services (CMS) fee-for-service Medicare data and four US commercial claims databases. Patients with a diagnosis code for obesity or for BMI ≥ 30 kg/m2 who were newly initiated on apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013 and September 30, 2015 were included. Patients were propensity score matched by treatment (DOAC vs DOAC and DOAC vs warfarin) in each database, and the results were pooled. Cox models were used to evaluate the risks of stroke or SE and MB. A total of 88,461 patients with obesity were included in the study. Apixaban was associated with a significantly lower risk of stroke or SE compared with warfarin (hazard ratio [HR] 0.63, 95% confidence interval [CI] 0.49–0.82) (Fig. 6). Apixaban had a lower risk of MB compared with warfarin (HR 0.54, 95% CI 0.49–0.61) (Fig. 6). Compared to rivaroxaban, apixaban was associated with a lower risk of stroke or SE (HR 0.78, 95% CI 0.64–0.94) and MB (HR 0.52, 95% CI 0.47–0.59). Compared to dabigatran, apixaban had a non-significant difference for the risk of stroke or SE (HR 0.71, 95% CI 0.49–1.04) and a lower risk of MB (HR 0.78, 95% CI 0.61–0.99).

Reproduced from Deitelzweig S, Keshishian A, Kang A, et al. Effectiveness and Safety of Oral Anticoagulants among NVAF Patients with Obesity: Insights from the ARISTOPHANES Study. J Clin Med. 2020;9:1633 [89], under Creative Commons license 4.0 (CC BY-4.0)
Incidence rates and hazard ratios for stroke or SE and MB among DOACs vs warfarin in obese patients with a diagnosis code for BMI ≥ 30 kg/m2. BMI body mass index, CI confidence interval, DOAC direct oral anticoagulant, GI gastrointestinal, ICH intracranial hemorrhage, MB major bleeding, NOAC non–vitamin K antagonist oral anticoagulant, ref reference, SE systemic embolism.
A subanalysis using the same methodology as the main ARISTOPHANES obesity analysis was conducted in patients who were severely obese (data table not shown) [89]. Severe obesity was defined using diagnosis codes indicating morbid obesity or a BMI ≥ 40 kg/m2, and patients were re-matched. Among all patients with obesity in the pooled sample, 39.5% were identified as being severely obese. Propensity score matching resulted in 6310 apixaban–warfarin pairs of patients. There was no significant difference in the risk of stroke or SE with apixaban versus warfarin or between apixaban versus rivaroxaban or apixaban versus dabigatran. Apixaban had a lower risk of MB compared to warfarin, dabigatran, and rivaroxaban (Fig. 6).
6.3 VTE
A recent study pooled five US healthcare claims databases to evaluate the risk of recurrent VTE, MB, and CRNM bleeding among patients with VTE, stratified by obesity [90]. This study utilized pooled data from the CMS fee-for-service Medicare data and four US commercial claims databases. Patients with one or more medical claims for VTE in any position (index VTE event) in the inpatient or outpatient setting were identified from September 1, 2014 until the end of available data. Adults (≥ 18 years in commercial databases and ≥ 65 years in the Medicare database) were selected if they had one or more pharmacy claims for warfarin or apixaban during the 30-day period following the index VTE event. The first warfarin or apixaban prescription date was designated as the index date. Stabilized inverse probability treatment weighting (IPTW) was conducted to balance patient characteristics between the treatment cohorts within each database. After pooling post-IPTW cohorts from the five databases, subgroup interaction analysis was conducted to evaluate whether the treatment effects were consistent across patients with and without obesity. Obesity and severe obesity were identified based on diagnosis codes that indicated obesity or codes that indicated a BMI ≥ 30 or ≥ 40 kg/m2, respectively. Cox proportional hazard models were used to evaluate the risk of recurrent VTE, MB, and CRNM bleeding [90].
A total of 112,024 non-obese patients and 43,095 obese patients were identified, of whom 19,751 were morbidly obese. In the IPTW population, no significant interactions were observed between the treatment effects of apixaban versus warfarin and obesity status for recurrent VTE or MB (both Pinteraction > 0.10; Fig 7). In both obese and morbidly obese patients, apixaban was associated with a significantly lower risk of recurrent VTE (obese: HR 0.73, 95% CI 0.64–0.84; morbidly obese: HR 0.65, 95% CI 0.53–0.80) and of MB (obese: HR 0.73 95% CI 0.62–0.85; morbidly obese: HR 0.68, 95% CI 0.54–0.86) compared with warfarin.

Reproduced from Cohen A, Sah J, Lee T, et al. Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity. J Clin Med. 2021;10:200 [90], under Creative Commons license 4.0 (CC BY-4.0)
Evaluation of recurrent VTE, MB, and CRNM bleeding among patients with VTE who initiated apixaban or warfarin, stratified by body weight category [90]. CRNM clinically relevant non-major, CI confidence interval, IR incidence rate, MB major bleeding, VTE venous thromboembolism.
6.4 NVAF or VTE
In a single-center (Montefiore Medical Center, Bronx, NY, USA), retrospective chart analysis, the efficacy and safety of apixaban and rivaroxaban were compared with warfarin in severely obese patients with NVAF or VTE [91]. The study included 795 patients who were ≥ 18 years with a BMI ≥ 40 kg/m2 and who were prescribed apixaban (n = 150), rivaroxaban (n = 326), or warfarin (n = 319) for either VTE or NVAF between March 1, 2013 and March 1, 2017. Patients who had both VTE and NVAF were excluded, as were patients with indications other than NVAF or VTE and those who were unable to confirm actual treatment start date or were missing follow-up after treatment initiation. In 366 patients prescribed an anticoagulant for VTE, the incidence of recurrent VTE was similar between the apixaban (1/47; 2.1%) and warfarin (2/167; 1.2%) cohorts (P = 0.74). The incidence of MB in this patient group was also similar between the treatment cohorts (apixaban: 1/47 [2.1%], warfarin: 4/167 [2.4%]; P = 0.77). In 429 patients who were prescribed an anticoagulant for NVAF, the incidence of stroke or SE was similar between the treatment cohorts (apixaban: 1/103 [1.0%], warfarin: 2/152 [1.3%]; P = 0.71). MB was also similar between treatment groups in patients with NVAF (apixaban: 3/103 [2.9%], warfarin: 12/152 [7.9%]; P = 0.063). Time-to-event analyses showed that the risks of all outcomes in patients with VTE, and stroke and composite bleeding in patients with NVAF, were similar between the anticoagulant cohorts. In a subgroup of 100 patients with NVAF and a BMI ≥ 50 kg/m2, one of 19 patients (5.3%) taking apixaban experienced a stroke, whereas one of the 44 patients (2.3%) taking warfarin had a stroke (P = 0.48). The incidence of both MB and composite bleeding was comparable among the anticoagulants.
7 Summary
This review comprehensively summarizes available data on the benefits and risks of apixaban in healthy volunteers and obese patients across PK studies, RCTs, and observational studies.
Although approved US and EU labeling do not stipulate dose adjustment, or avoidance, in patients at the higher end of the weight spectrum, some earlier consensus guidelines suggest that DOACs, including apixaban, should not be used in patients weighing > 120 kg. This is due to a perceived relative paucity of clinical data in these patients, and the hypothetical potential for under-dosing [36, 75,76,77, 80, 81, 83,84,85,86]. The 2019 focused update by the AHA/ACC/HRS suggested checking DOAC levels in patients with a BMI > 35–40 kg/m2 or weight > 120 kg. [35]. However, there are no defined therapeutic ranges that predict efficacy or safety outcomes for apixaban (or other DOACs). A recent (2021) update of the ISTH guidelines, based on the availability of additional data, states that standard doses of apixaban or rivaroxaban, along with VKAs, weight-based LMWH, and fondaparinux, are among appropriate anticoagulation options, regardless of high BMI and weight for the treatment of VTE and VTE prophylaxis after hip or knee replacement surgery [29]. It is no longer suggested to regularly follow peak or trough drug-specific DOAC levels, because there are insufficient data to influence management decisions.
Based on PK studies and RCT data at the time of approval, the apixaban US PI does not require dose adjustments in patients with high weight alone. Post-approval analyses of RCTs, and observational studies, are consistent with the US PI dose recommendation.
In conclusion, the accumulated body of data from RCTs and observational studies suggests that the benefit-risk profile of apixaban in obese patients is similar to that of patients with normal body weights. Future studies should further clarify the relationship between obesity and outcomes especially in those with extreme weights, > 140–150 kg or BMI > 50 kg/m2, where data are currently limited.
Change history
04 June 2022
A peer-reviewed video was retrospectively added to this publication.
References
Kearns K, Dee A, Fitzgerald AP, Doherty E, Perry IJ. Chronic disease burden associated with overweight and obesity in Ireland: the effects of a small BMI reduction at population level. BMC Public Health. 2014;14:143.
World Health Organization. WHO obesity and overweight fact sheet. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 31 Aug 2020.
Centers for Disease Control and Prevention. Defining adult overweight & obesity. 2021. https://www.cdc.gov/obesity/adult/defining.html. Accessed 29 Mar 2021.
Rocca B, Fox KAA, Ajjan RA, Andreotti F, Baigent C, Collet JP, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J. 2018;39(19):1672–86.
University of Rochester. What is morbid obesity? 2020. https://www.urmc.rochester.edu/highland/bariatric-surgery-center/journey/morbid-obesity.aspx. Accessed 24 June 2021.
ICD10Data.com. Morbid (severe) obesity due to excess calories. 2021. https://www.icd10data.com/ICD10CM/Codes/E00-E89/E65-E68/E66-/E66.01. Accessed 26 Apr 2021.
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8.
Fryar CD, Carroll, M.D., Ogden, C.L. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2015–2016. 2018. https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm. Accessed 22 Sep 2021.
Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2011–2014. Vital Health Stat. 2016;33(39):1–46.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89.
Wang J, Yang YM, Zhu J, Zhang H, Shao XH. Obesity paradox in patients with atrial fibrillation and heart failure. Int J Cardiol. 2014;176(3):1356–8.
Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016;37(38):2869–78.
Wang SY, Giugliano RP. Non-vitamin K antagonist oral anticoagulant for atrial fibrillation in obese patients. Am J Cardiol. 2020;127:176–83.
Sandhu RK, Ezekowitz JA, Hijazi Z, Westerbergh J, Aulin J, Alexander JH, et al. Obesity paradox on outcome in atrial fibrillation maintained even considering the prognostic influence of biomarkers: insights from the ARISTOTLE trial. Open Heart. 2018;5(2):908.
Liu X, Guo L, Xiao K, Zhu W, Liu M, Wan R, et al. The obesity paradox for outcomes in atrial fibrillation: evidence from an exposure-effect analysis of prospective studies. Obes Rev. 2020;21(3):e12970.
Foy AJ, Mandrola J, Liu G, Naccarelli GV. Relation of obesity to new-onset atrial fibrillation and atrial flutter in adults. Am J Cardiol. 2018;121(9):1072–5.
Cohoon KP, McBane RD, Ammash N, Slusser JP, Grill DE, Wysokinski WE. Relationship between body mass index and left atrial appendage thrombus in nonvalvular atrial fibrillation. J Thromb Thrombolysis. 2016;41(4):613–8.
Zhang XX, Wei M, Shang LX, Lu YM, Zhang L, Li YD, et al. LDL-C/HDL-C is associated with ischaemic stroke in patients with non-valvular atrial fibrillation: a case-control study. Lipids Health Dis. 2020;19(1):217.
Li X, Zuo C, Ji Q, Xue Y, Wang Z, Lv Q. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931–43.
Cho BH, Cheon K, Lee KY, Jung YH, Han SW, Park JH, et al. Association between body mass index and stroke severity in acute ischaemic stroke with non-valvular atrial fibrillation. Eur J Neurol. 2020;27(8):1672–9.
Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28–36.
Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630–6.
Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179(5):417–26.
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102.
Rodger MA, Le Gal G, Anderson DR, Schmidt J, Pernod G, Kahn SR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7.
Connolly GC, Khorana AA. Risk stratification for cancer-associated venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):35–47.
Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. 2012;10(6):1019–25.
Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19(8):1874–82.
Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308–13.
Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206–32.
Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):2247–59.
Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927–74.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–76.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32.
Martin AC, Thomas W, Mahir Z, Crowley MP, Dowling T, Breen K, et al. Direct oral anticoagulant concentrations in obese and high body weight patients: a cohort study. Thromb Haemost. 2021;121(2):224–33.
Falk K, McComb MN, Shapiro NL, Uppuluri EM. Prescribing pattern of oral anticoagulants in patients with obesity. J Pharm Pract. 2020; p. 897190020969276.
Boriani G, Huisman MV, Teutsch C, Marler S, Franca LR, Lu S, et al. Influence of BMI and geographical region on prescription of oral anticoagulants in newly diagnosed atrial fibrillation: the GLORIA-AF Registry Program. Eur J Intern Med. 2020;80:35–44.
Bristol-Myers Squibb. Eliquis® (apixaban tablets). Prescribing information. 2019. https://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 21 July 2021.
Upreti VV, Song Y, Wang J, Byon W, Boyd RA, Pursley JM, et al. Effect of famotidine on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Clin Pharmacol. 2013;5(1):59–66.
Bristol-Myers Squibb. Eliquis® (apixaban tablets). Summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 21 July 2021.
Byon W, Garonzik S, Boyd RA, Frost CE. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–79.
Instrumentation Laboratory. The first direct oral anticoagulant test authorized for clinical use on automated hemostasis systems. https://www.instrumentationlaboratory.com/en/instrumentation-laboratory-receives-us-fda-marketing-authorization-first-apixaban-diagnostic-test. Accessed 8 Feb 2021.
Linguamatics. What is I2E, Linguamatics Natural Language Processing (NLP) Platform? 2021. https://www.linguamatics.com/products/i2e. Accessed 19 Feb 2021.
McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–21.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–9.
Agnelli G, Becattini C, Meyer G, Munoz A, Huisman MV, Connors JM, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–607.
Windecker S, Lopes RD, Massaro T, Jones-Burton C, Granger CB, Aronson R, et al. Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome treated medically or with percutaneous coronary intervention or undergoing elective percutaneous coronary intervention: insights from the AUGUSTUS trial. Circulation. 2019;140(23):1921–32.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31.
Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908–16.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Frost C, Song Y, Barrett YC, Wang J, Pursley J, Boyd RA, et al. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;6:179–87.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek E, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Cirincione B, Kowalski K, Nielsen J, Roy A, Thanneer N, Byon W, et al. Population pharmacokinetics of apixaban in subjects with non-valvular atrial fibrillation. CPT Pharmacomet Syst Pharmacol. 2018;7(11):728–38.
Byon W, Sweeney K, Frost C, Boyd R. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacomet Syst Pharmacol. 2017;6(5):340–9.
Cohen AT, Pan S, Byon W, Ilyas BS, Taylor T, Lee TC. Efficacy, safety, and exposure of apixaban in patients with high body weight or obesity and venous thromboembolism: insights from AMPLIFY. Adv Ther. 2021;38(6):3003–18.
Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–50.
Wasan SM, Feland N, Grant R, Aston CE. Validation of apixaban anti-factor Xa assay and impact of body weight. Thromb Res. 2019;182:51–5.
Kido K, Lee JC, Hellwig T, Gulseth MP. Use of direct oral anticoagulants in morbidly obese patients. Pharmacotherapy. 2020;40(1):72–83.
Kido K, Shimizu M, Shiga T, Hashiguchi M. Meta-analysis comparing direct oral anticoagulants versus warfarin in morbidly obese patients with atrial fibrillation. Am J Cardiol. 2020;126:23–8.
Boonyawat K, Caron F, Li A, Chai-Adisaksopha C, Lim W, Iorio A, et al. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(7):1322–33.
Zhou Y, Ma J, Zhu W. Efficacy and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20(1):51–60.
Mai V, Marceau-Ferron E, Bertoletti L, Lacasse Y, Bonnet S, Lega JC, et al. Direct oral anticoagulants in the treatment of acute venous thromboembolism in patients with obesity: a systematic review with meta-analysis. Pharmacol Res. 2021;163:105317.
Elshafei MN, Mohamed MFH, El-Bardissy A, Ahmed MB, Abdallah I, Elewa H, et al. Comparative effectiveness and safety of direct oral anticoagulants compared to warfarin in morbidly obese patients with acute venous thromboembolism: systematic review and a meta-analysis. J Thromb Thrombolysis. 2020;51(2):388–96.
Pathak R, Karmacharya P, Giri S, Poudel DR, Aryal MR, Bhatt VR, et al. Meta-analysis on efficacy and safety of new oral anticoagulants for venous thromboembolism prophylaxis in overweight and obese postarthroplasty patients. Blood Coagul Fibrinolysis. 2015;26(6):635–42.
Buck MM, Haddon AM, Paneccasio A, Skoloda DJ, Zimmerman DE, Guarascio AJ, et al. Safety and efficacy of rivaroxaban and apixaban in patients with increased body mass: a systematic review. Clin Drug Investig. 2021;41(4):353–69.
Hohnloser SH, Fudim M, Alexander JH, Wojdyla DM, Ezekowitz JA, Hanna M, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation. 2019;139(20):2292–300.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807–15.
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–98.
Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez LM, Ramacciotti E, et al. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J Thromb Haemost. 2013;11(3):444–51.
Leven C, Hoffmann C, Roche C, Couturaud F, Thereaux J, Lacut K. Impact of bariatric surgery on oral anticoagulants pharmacology, and consequences for clinical practice: a narrative review. Fundam Clin Pharmacol. 2021;35(1):53–61.
Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother. 2019;53(2):165–70.
Kaplan RM, Diaz CL, Strzelczyk T, You C, Saour B, Fine M, et al. Outcomes with novel oral anticoagulants in obese patients who underwent electrical cardioversion for atrial tachyarrhythmias. Am J Cardiol. 2018;122(7):1175–8.
Lucijanic M, Jurin I, Jurin H, Lucijanic T, Starcevic B, Skelin M, et al. Patients with higher body mass index treated with direct / novel oral anticoagulants (DOAC / NOAC) for atrial fibrillation experience worse clinical outcomes. Int J Cardiol. 2020;301:90–5.
Kaplan RM, Tanaka Y, Passman RS, Fine M, Rasmussen-Torvik LJ, Vupputuri S, et al. Efficacy and safety of direct oral anticoagulants for atrial fibrillation across body mass index categories. J Am Heart Assoc. 2020;9(24):e017383.
Wiethorn EE, Bell CM, Wiggins BS. Effectiveness and safety of direct oral anticoagulants in patients with nonvalvular atrial fibrillation and weighing ≥ 120 kilograms versus 60-120 kilograms. Am J Cardiovasc Drugs. 2021;21(5):545–51.
Younis M, Elkaryoni A, Williams GW II, Jakhar I, Suman S, Simon S, et al. The use of direct oral anticoagulants in the management of venous thromboembolism in patients with obesity. Cureus. 2020;12(8):e1006.
Quan S, Smith J, Wu C, Koshman SL, Nguyen B, Bungard TJ. Anticoagulant therapies and outcomes in obese patients with acute venous thromboembolism. Thromb Res. 2020;187:56–62.
Lachant DJ, Bach C, Fe A, White RJ, Lachant NA. Direct oral anticoagulant therapy in patients with morbid obesity after intermediate- or high-risk pulmonary emboli. ERJ Open Res. 2021;7(1):00554-2020.
Choi Y, Kushnir M, Heisler Billett H. Apixaban is safe and effective in morbidly obese patients: a retrospective analysis of 390 patients with BMI ≥40. Blood. 2017;130(Suppl 1)(1105).
Doucette K, Latif H, Vakiti A, Tefera E, Patel B, Fitzpatrick K. Efficacy and safety of direct-acting oral anticoagulants (DOACs) in the overweight and obese. Adv Hematol. 2020;2020:3890706.
Kalani C, Awudi E, Alexander T, Udeani G, Surani S. Evaluation of the efficacy of direct oral anticoagulants (DOACs) in comparison to warfarin in morbidly obese patients. Hosp Pract (1995). 2019;47(4):181–5.
Patil T, Lebrecht M. A single center retrospective cohort study evaluating use of direct oral anticoagulants (DOACs) in morbidly obese veteran population. Thromb Res. 2020;192:124–30.
Jain R, Watzker A, Luo X, Kang AL, Baker CL, Rosenblatt L, et al. Validation of obesity coding among newly treated nonvalvular atrial fibrillation patients using an integrated electronic medical record and claims database. Curr Med Res Opin. 2020;36(2):189–97.
Briasoulis A, Mentias A, Mazur A, Alvarez P, Leira EC, Vaughan Sarrazin MS. Comparative effectiveness and safety of direct oral anticoagulants in obese patients with atrial fibrillation. Cardiovasc Drugs Ther. 2021;35(2):261–72.
Deitelzweig S, Keshishian A, Kang A, Dhamane AD, Luo X, Li X, et al. Effectiveness and safety of oral anticoagulants among NVAF patients with obesity: insights from the ARISTOPHANES study. J Clin Med. 2020;9(6):1633.
Cohen A, Sah J, Lee T, Rosenblatt L, Hlavacek P, Emir B, et al. Effectiveness and safety of apixaban vs. warfarin in venous thromboembolism patients with obesity and morbid obesity. J Clin Med. 2021;10(2):200.
Kushnir M, Choi Y, Eisenberg R, Rao D, Tolu S, Gao J, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre, retrospective analysis of chart data. Lancet Haematol. 2019;6(7):e359–65.
Acknowledgments
Medical writing support was provided by Claire Line, PhD, of Caudex, and technical editorial assistance was provided by Marcella De Simone of Caudex, both funded by Bristol Myers Squibb and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The development of this manuscript was funded by Bristol Myers Squibb and Pfizer.
Conflict of interest
MJJ, WB, RWD, AJQ, and CR are employees and shareholders of Pfizer. MC is an employee of Pfizer. PG, JO, and SJM are employees and shareholders of Bristol Myers Squibb.
Availability of data and material
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics approval
Not applicable.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
MJ had the original concept for the article, and all authors agreed on its scope and methodology. The literature search logic and methodology were developed by AQ and MC, who also conducted the search. All articles in the search output were reviewed by RD, JO, and MJ. WB and SJM wrote the pharmacokinetics section; RD wrote the retrospective observational data section; AQ and MC wrote the literature search methodology section; MJ wrote all other sections. All authors critically reviewed the work in its entirety and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jamieson, M.J., Byon, W., Dettloff, R.W. et al. Apixaban Use in Obese Patients: A Review of the Pharmacokinetic, Interventional, and Observational Study Data. Am J Cardiovasc Drugs 22, 615–631 (2022). https://doi.org/10.1007/s40256-022-00524-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00524-x