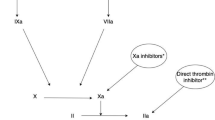
Abstract
Direct oral anticoagulants (DOACs) have been developed as a viable and in some cases superior alternative to warfarin. These agents have overcome some of the limitations of warfarin, which has a narrow therapeutic window and many food and drug interactions. DOACs have been demonstrated to have a more predictable and reliable pharmacology and, unlike warfarin, do not require frequent monitoring of anticoagulant effect. For these reasons, the use of DOACs is increasing. Despite the many positive attributes of these agents, limitations and contraindications do exist. An understanding of the pharmacology, indications, and contraindications is therefore crucial for effective patient management. We review the available agents to aid in effective drug utilization.

Similar content being viewed by others
References
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Gibson CM, Halaby R, Korjian S, et al. The safety and efficacy of full- versus reduced-dose betrixaban in the acute medically ill VTE (venous thromboembolism) prevention with extended-duration betrixaban (APEX) trial. Am Heart J. 2017;185:93–100.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet. 2014;383(9921):955–62.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962.
January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2019. https://doi.org/10.1016/j.jacc.2019.01.011.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300–5.
Baetz BE, Spinler SA. Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy. 2008;28(11):1354–73.
Spinler Sarah. The pharmacology and therapeutic use of dabigatran etexilate. J Clin Pharmacol. 2013;53(1):1–13.
Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303.
Pradaxa [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2017.
Vazquez Sara. Drug–drug interactions in an ear of multiple anticoagulants: a focus on clinically relevant drug interactions. Blood. 2018;132(21):2230–9.
“Dabigatran Drug Interaction Potential”. University of Washington. 2014. http://www.depts.washington.edu/anticoag/home/content/dabigatran-drug-interaction-potential. Accessed 9 Feb 2019.
Todd Crump “Straight Healthcare”. http://www.straighthealthcare.com. Accessed 10 Feb 2019.
Gosselin RC, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost. 2016;14(5):886–93.
Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209–19.
Julia S, James U. Direct oral anticoagulants: a quick guide. Eur Cardiol Rev. 2017;12(1):40–5.
Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70(24):3042–67.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93.
Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69.
“Summery of Product Characteristics”. European Medicines Agency, European Union agencies network. 2018. http://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa#product-information-section%20pr. Accessed 9 Feb 2019.
Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–52.
Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–18.
Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370(9591):949–56.
Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–14.
Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(10):530–9.
Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5(2):e002776.
Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011;364(19):1788–90.
Moore TJ, Cohen MR, Mattison DR. Dabigatran, bleeding, and the regulators. BMJ. 2014;23(349):g4517.
Safouris A, Triantafyllou N, Parissis J, Tsivgoulis G. The case for dosing dabigatran: how tailoring dose to patient renal function, weight and age could improve the benefit–risk ratio. Ther Adv Neurol Disord. 2015;8(6):245–54.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76.
Schulman S, Carrier M, Lee AY, et al. Perioperative management of dabigatran a prospective cohort study. Circulation. 2015;132(3):167–73.
Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343–8.
Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69(7):871–98.
Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377(5):431–41.
Stacy ZA, Call WB, Hartmann AP, Peters GL, Richter SK. Edoxaban: a comprehensive review of the pharmacology and clinical data for the management of atrial fibrillation and venous thromboembolism. Cardiol Ther. 2016;5(1):1–18.
Andexxa (andexant alfa) [prescribing information]. South San Francisco, CA: Portola Pharmaceuticals Inc; 2018.
Connolly SJ, Milling TJ Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with Factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–41.
Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of Factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–24.
Connors JM. Antidote for Factor Xa anticoagulants. N Engl J Med. 2015;373(25):2471–2.
Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37(1):74–81.
Frost C, Wang J, Nepal S, et al. Apixaban, an oral, direct Factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476–87.
“Apixaban Drug Interaction Potential”. University of Washington. 2014. http://www.depts.washington.edu/anticoag/home/content/apixaban-drug-interaction-potential. Accessed 10 Feb 2019.
Eliquis (apixaban) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2017.
Cabral KP. Pharmacology of the new target-specific oral anticoagulants. J Thromb Thrombolysis. 2013;36(2):133–40.
Martin A, Stewart R. Safety and efficacy of apixaban in the treatment of atrial fibrillation. Clin Med Insights Cardiol. 2012;6:103–9.
Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807–15.
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–98.
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Avezum A, Lopes RD, Schulte PJ, et al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the ARISTOTLE trial. Circulation. 2015;132(8):624–32.
Lassen MR, Davidson BL, Gallus A, et al. The efficacy and safety of apixaban, an oral, direct Factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost. 2007;5(12):2368–75.
Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a Factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76(5):776–86.
Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37(4):412–9.
Xarelto (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2019.
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct Factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–21.
EINSTEIN Investigators, Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510.
EINSTEIN–PE Investigators, Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–97.
Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211–22.
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–75.
Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372(9632):31–9.
Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776–86.
Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–30.
Eriksson BI, Borris LC, Dahl OE, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation. 2006;114(22):2374–81.
Savaysa (edoxaban) [prescribing information]. Parsippany, NJ: Daiichi Sankyo; 2016.
Lip GY, Agnelli G. Edoxaban: a focused review of its clinical pharmacology. Eur Heart J. 2014;35(28):1844–55.
Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–15.
Carnicelli AP, De Caterina R, Halperin JL, et al. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135(13):1273–5.
Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134(1):24–36.
Weitz JI, Connolly SJ, Patel I, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral Factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104(3):633–41.
Bevyxxa (betrixaban) [prescribing information]. South San Francisco, CA: Portola Pharmaceuticals Inc; 2017.
Chan NC, Bhagirath V, Eikelboom JW. Profile of betrixaban and its potential in the prevention and treatment of venous thromboembolism. Vasc Health Risk Manag. 2015;11:343–51.
Turpie AG, Bauer KA, Davidson BL, et al. A randomized evaluation of betrixaban, an oral Factor Xa inhibitor, for prevention of thromboembolic events after total knee. Thromb Haemost. 2009;101(1):68–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TK, CH, JH, TC, AK, HT, BM, AJ, and AH have no conflicts of interest that are relevant to the content of this review.
Funding
No sources of funding were used to assist with the preparation of this review.
Rights and permissions
About this article
Cite this article
Khachatryan, T., Hauschild, C., Hoff, J. et al. Review of Direct Oral Anticoagulants and Guide for Effective Drug Utilization. Am J Cardiovasc Drugs 19, 525–539 (2019). https://doi.org/10.1007/s40256-019-00344-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00344-6