Abstract
Purpose
The OPTIMISE study uses a Multiphase Optimisation Strategy (MOST) to identify the best combination of four interventions targeting key diabetes self-care behaviours for use in clinical practice to improve short-term glycaemic outcomes.
Methods
This 4-week intervention trial will recruit 80 young people (aged 13–20 years) with type 1 diabetes ≥ 6 months duration), and pre-enrolment HbA1c ≥ 58 mmol/mol (7.5%) in the prior 6 months. Both main intervention and interaction effects will be estimated using a linear regression model with change in glucose time-in-range (TIR; 3.9–10.0 mmol/L) as the primary outcome. Participants will be randomised to one of 16 conditions in a factorial design using four intervention components: (1) real-time continuous glucose monitoring (CGM), (2) targeted snacking education, (3) individualised sleep extension, and (4) values-guided self-care goal setting. Baseline and post-intervention glucose TIR will be assessed with blinded CGM. Changes in self-care (snacking behaviours, sleep habits and duration, and psychosocial outcomes) will be assessed at baseline and post-intervention to determine if these interventions impacted behaviour change.
Discussion
The study outcomes will enable the selection of effective and efficient intervention components that increase glucose TIR in young people who struggle to achieve targets for glycaemic control. The optimised intervention will be evaluated in a future randomised controlled trial and guide the planning of effective clinical interventions in adolescents and young adults living with type 1 diabetes.
Trial registration
This trial was prospectively registered with the Australian New Zealand Clinical Trials Registry on 7 October 2020 (ACTRN12620001017910) and the World Health Organisation International Clinical Trails Registry Platform on 26 July 2020 (Universal Trial Number WHO U1111-1256-1248).
Similar content being viewed by others
Background
Type 1 diabetes (T1D) is the most common form of diabetes in young people [1]. Current guidelines recommend a target HbA1c < 53 mmol/mol (< 7.0%) [2] and interstitial glucose target of 70% time-in-range (TIR: 3.9–10.0 mmol/L) [3] to prevent long-term diabetes complications [4]. Despite advances in treatment and innovations in diabetes technology, glycaemic control deteriorates during the transition from childhood to young adulthood [5]. Systematic reviews of intervention trials to improve clinical and psychosocial outcomes in young people with type 1 diabetes suggest multi-component interventions may be more effective than single-component interventions among adolescents [6], although the evidence for young adults is limited [7].
A multiphase optimisation strategy (MOST) is a systematic method used to develop effective, robust, and scalable behavioural interventions [8]. MOST consists of three stages: (1) preparation, (2) optimisation and (3) evaluation of the optimised intervention in an RCT [8]. The present study focuses on optimisation, in which candidate intervention components that were carefully selected during the preparation phase will be evaluated against a clinically meaningful performance criterion. The combination of components that achieve the greatest improvement in TIR will comprise the ‘optimised’ intervention package. The effectiveness of the optimised intervention will then be evaluated in a future standard randomised controlled trial.
As part of the preparation phase, four candidate components were selected to target diabetes self-care behaviours that are important for young people to achieve glycaemic control targets. The conceptual model underlying the intervention is presented in Fig. 1.
Continuous glucose monitoring
Glucose monitoring 6–10 times each day is recommended to inform treatment decisions and subsequently glycaemic control [9]. Adherence to multiple finger-prick tests per day is difficult [10]. Real-time continuous glucose monitoring (rtCGM) increases TIR and reduces HbA1c among adolescents and young adults [11]. A synergistic effect of glucose monitoring technology use with additional self-care support warrants exploration [12].
Snacking habits
Skipping meals, increased or inconsistent snacking behaviours (or ‘grazing’), and insulin omission with snacks contribute to suboptimal glycaemic control [13]. Educating young people to take insulin boluses for snacks is associated with healthier glycaemic control [14]; however, no interventions have targeted incorporating planned snacks into a routine meal plan. This study will explore a pragmatic intervention to extend previous research on snacking.
Sleep extension
Recent research highlights sleep as an important target for improving glycaemic control among youth with type 1 diabetes [15]. There is likely a bidirectional relationship between sleep and glycaemic control [16], with sleep disrupted by variability in timing due to night-time self-care, later bedtimes as adolescents mature, and early waking times dictated by school and work schedules. Sleep extension is feasible in youth (ages 10–16 yrs) [17] and warrants further investigation in older youth.
Values-guided diabetes self-management
Values-guided diabetes self-management encourages personal choice, which is ideally suited to adolescence and young adulthood when individuals are becoming increasingly autonomous. Values-guided self-management is theorised to motivate youth to accept the negative aspects of their condition (e.g., pain, perceived stigma, undesirable glucose values) and adhere to treatment because doing so is acting in accordance with the person one wants to be or contributing to a more meaningful life instead of ‘the numbers’ (HbA1c targets) [18]. This approach can support self-management among adults with type 2 diabetes [19] and may improve quality of life among adolescents with a chronic condition [20].
This study aims to evaluate the effectiveness of four individual intervention components to (1) estimate the individual and synergistic effects of the four individual interventions on glucose TIR and determine which combination(s) offer the greatest improvements, (2) explore potential mediators and moderators of the efficacy of each of the four individual intervention components, and (3) provide evidence for an intervention or combination that optimises outcomes for youth with type 1 diabetes.
Methods
Study design
The proposed research is an optimisation trial using a 24 factorial experimental study design (Table 1) [8]. This design is more economical compared to conducting four randomised controlled trials of the separate components because fewer participants are required to achieve the same power [8]. Using a correlation between measures of 0.6 and a standard deviation of 10 (based on previously published data) [12] a sample size of 80 is required. This sample size (5 participants in each of the 16 groups and 40 assigned to each component – see Table 1) gives 80% power at α = 0.05 level to detect an improvement of 5% TIR. Using effect coding, the interaction terms (which assess combinations of intervention components) are equally powered to the main effects.
Study procedures
Participant eligibility and recruitment
The research is being conducted across four academic centres (University of Otago – Dunedin, Christchurch, and Wellington campuses, and The University of Auckland) with established strong collaborations with District Health Boards (DHBs) covering approximately three-quarters of New Zealand (NZ)’s total population.
Participants will be invited to participate during a routine clinic visit by diabetes care providers affiliated with the academic centres and advertisements through NZ diabetes organisations’ social media accounts and websites. Eligibility criteria include: age 13–20 years, inclusive; type 1 diabetes for at least six months; mean HbA1c ≥ 58 mmol/mol (7.5%) in the prior six months. Exclusion criteria include: severe diabetes-related complications (e.g., nephropathy on treatment), severe depression requiring treatment, diagnosed sleep or eating disorders under active treatment; habitual sleep duration > 10 h; shift worker (works or has plans to work at least 3 h between midnight and 0500 h during the intervention period); current use of rtCGM technology (use of intermittently scanned CGM not excluded).
Intervention component procedures
Each component will aim to facilitate behaviour change during a 4-week intervention phase using minimal resources. Components will be delivered 1:1 by trained research staff (in person or via Zoom). A full description of components is available in the supplemental file. Protocols were developed to standardise component delivery with careful consideration of the burden for youth allocated to the intervention and staff delivering it. A pragmatic approach guided component content and delivery to maximise the likelihood the optimised intervention will be acceptable and scalable across NZ.
Glucose monitoring
The glucose monitoring component will use the Dexcom® G6 CGM system (Dexcom, Inc., San Diego, CA, USA) linked to a personal mobile device. The system collects glucose data every five minutes, is approved for making diabetes treatment decisions without confirmatory finger-prick tests and facilitates glucose data sharing. The CGM component also consists of personalised feedback on recent CGM data, goal setting, and a 2-week review of CGM data.
Snacking habits
The snacking component consists of written, verbal, and visual information about healthful snacking, an action planning activity that if adopted is likely to improve snacking habits, and a 2-week review of the snacking goal identified in the action planning activity. In this research, a ‘snack’ is defined as a food or drink (not including water) consumed between main meals and does not include treatment for hypoglycaemia. The snacking component was developed by S.R., C.E.S., R.T. and S.E.S.
Sleep extension
Participants allocated to the sleep extension component will be encouraged to go to bed one hour earlier than their usual bedtime on weekdays and weekends (as determined by their baseline self-reported ‘lights out’ time), while maintaining their usual wake-up time. Sleep hygiene education will be provided to support participants in achieving the goal. The sleep extension goal will be reviewed at the 2-week follow-up visit. The protocol was adapted from B.C.G.’s previous research.
Values-guided diabetes self-management
The values-guided diabetes self-management component will comprise a 30–60 min psychoeducational session delivered 1:1 by a researcher (S.E.S.) who completed advanced workshops in Acceptance and Commitment Therapy (ACT) [21] and He Puna Whakaata (a values-based mātauranga Māori [Māori knowledge] programme for practitioners). The session will follow a protocol inspired by a brief values-informed healthy lifestyle intervention for young adults and the DNA-V (i.e., Discoverer, Noticer, Advisor and Values) model of behavioural interventions for youth [22], which focuses on a participant’s self-chosen diabetes-specific problem and an action planning activity that if adopted would be likely to improve the problem. A psychoeducational handout based on the DNA-V model will provide strategies for overcoming psychological barriers to self-management. The action plan will be reviewed at the 2-week follow-up visit. Expert review of this component was undertaken by clinical psychologists (A.C., M.K.) to ensure fidelity to the underpinning theoretical basis for the intervention and cultural appropriateness.
Outcome assessments
Outcomes to be assessed are outlined in Table 2.
Primary outcome
The FreeStyle Libre Pro (Abbott Diabetes Care, Witney, Oxon, U.K.) blinded CGM system will be used to measure interstitial glucose data for 14 days and assess change in TIR.
Secondary outcomes
Changes in self-care will be assessed in participants only (not parents) to determine if these interventions impacted behaviour change. The participant’s self-care data will be collected using electronic questionnaires administered via REDCap (Research Electronic Data Capture), a password protected secure web-based application hosted at the University of Otago.
Diabetes self-management
The Self-Care Inventory-Revised (SCI-R) [23] is a validated measure of perceived adherence to diabetes self-care recommendations among youth with diabetes. Responses to 15 items are given on a 5-point Likert type scale from 1-Never to 5-Always, with higher scores indicating more optimal self-care behaviours.
Snacking habits
A questionnaire developed by S.E.S., J.J.H., S.R., and C.E.S. to assess snacking behaviours over the previous seven days. Questions about the timing (between breakfast and lunch, between lunch and dinner, after dinner) and frequency (from never to most days) of planned snacks eaten as part of a routine meal plan are included, and self-reported caffeine and alcohol consumption before bed. Snacking behaviours during the school/workday and on weekends/during holidays will be reported separately and assessed for accuracy using the rtCGM traces. The frequency of grazing episodes (from none to 3 + per day) will be assessed using a question from a survey of adolescents and adults aged 15 + years [24]. A food frequency questionnaire will assess how often 13 categories of common snack foods (e.g., fruit, breakfast cereals, packaged snacks, rice/pasta/noodles) and beverages were consumed in the past seven days (from none to 3 + per day). The final questionnaire was modified based on their feedback. The snacking behaviours and snack foods questionnaires were pre-tested with a sample of young people with type 1 diabetes and modified based on their feedback.
Sleep habits and duration
Self-reported usual ‘lights out’, wake-up time, and hours of sleep on weeknights and weekend nights over the past seven days will be used to confirm eligibility and tailor recommendations to participants in the sleep extension component.
The Adolescent Sleep Hygiene Scale is a 33-item questionnaire to assess sleep-facilitating and sleep-inhibiting practices, with good internal consistency (Cronbach α = 0.80) [25]. Participants report how often each sleep item occurred over the last month along a 6-point ordinal rating scale. Higher scores indicate better sleep hygiene.
Participants will wear an ActiGraph (ActiGraph© wGT3X-BT, LLC, Florida, U.S.A.) on the non-dominant wrist for seven days and eight nights (continuously), to obtain objective sleep data. ActiGraphs will be initialised using 15s epochs. Participants will complete a sleep diary over the same period to record unusual or disturbed sleep to assist in interpreting sleep data. The actigraphy data will be downloaded into ActiLife Software (version 6.0 or later) and analysed with an automated script developed in MATLAB® (MathWorks, Natick, MA, USA). The script uses a count-scaled algorithm to estimate sleep onset and offset for sleep and awakenings. Participants will be excluded from the analysis if fewer than three valid nights of wear are obtained.
Valued living
The Valuing Questionnaire (VQ) [26] is a validated measure of how consistently an individual has been living with their self-determined values in the past week and is used to evaluate ACT interventions. The VQ contains 10-items rated from 0 (not at all) to 6 (completely true). Higher scores indicate more consistently living by one’s self-determined values.
Moderators
Baseline depression will be assessed using the Patient Health Questionnaire (PHQ) [27]. The PHQ-A will be administered to adolescents aged 13–17 years and the PHQ-9 will be administered to young adults ages 18 + years; the 9-item questionnaires answered on a 4-point Likert type scale from 0 (not at all) to 3 (nearly every day). Scores ≥ 15 suggest moderately severe to severe depression.
Baseline disordered eating will be assessed with the Diabetes Eating Problem Survey-Revised (DEPS-R); this 16-item tool is used to assess general and diabetes-specific disordered eating behaviours including weight loss, food restriction, insulin misuse, and vomiting [28]. The DEPS-R is scored on a 6-point Likert scale ranging from never to always, with scores ≥ 20 indicating the tendency towards disordered eating behaviours.
Mediators
The Diabetes Acceptance and Action Scale-Revised (DAAS-R) is a brief reliable and valid measure of acceptance of diabetes used in clinical and research settings among patients aged 16 years and older [29].
Both the Patient Reported Outcomes Measurement Information System (PROMIS)® Sleep-Related Impairment short form (v 1.0; 8a) [30] and the PROMIS Sleep Disturbance short form (v 1.0; 8a) [30] will be used to assess sleep-related impairment and disturbance, respectively, over the past seven days. Each questionnaire contains eight items, each item having five response options ranging in value from 1 to 5, with higher scores representing more of the concept being measured.
Data collection procedure
Demographic and clinical data
Demographic information will be self-reported via questionnaire or obtained from medical records including: age, gender, diabetes duration, height, weight, body mass index, insulin regimen total, self-identified ethnicity, employment or education status, living situation (e.g., 1- or 2-parent/caregiver household or flatting), address (to determine NZDep2018 score, a validated measure of socioeconomic status) [31]. Baseline HbA1c will be measured with a point-of-care device (DCA Vantage Analyzer, Siemens Healthcare Diagnostics, Ireland), linked directly to the Diabetes Control and Complications Trial method [certified through the National Glycohemoglobin Standardization Program (NGSP)].
Study visits
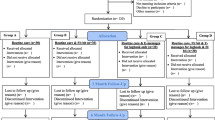
As shown in Fig. 2, four study visits will occur over six weeks. All staff involved in data collection and delivering interventions will be trained in the study procedures by the principal investigator (S.E.S.). To promote participant retention, study visits will be conducted in participants’ preferred location (i.e., at home or a private research space) and up to three contact attempts will be made via email, text message and/or phone call to schedule study visits.
Screening visit (day − 14)
Written informed consent will be obtained from participants aged 16–20 years and parents of those aged 13–15 years (written assent also obtained). Medical records will be reviewed to confirm the absence of exclusion criteria and mean of HbA1c results in the prior 6-months. Those who meet all inclusion, and no exclusion criteria, will be enrolled in the study and asked to apply a blinded CGM sensor and wear an ActiGraph initialised from the date of the screening visit.
Baseline visit (day 0)
Research staff will check the blinded CGM snapshot report for a minimum of 10 days of data and screen for overnight hypoglycaemia. Participants will then be randomised to one of 16 experimental conditions (i.e., 0 to 4 of the self-care components) for the intervention phase and each allocated intervention will commence, as outlined in the supplementary file. Participants will be randomly allocated to experimental conditions using a computer-generated list of random numbers uploaded to the REDCap randomisation module by a biostatistician (J.J.H.). Each experimental condition represents a different treatment protocol. The experimental condition is concealed until research staff initiate randomisation in REDCap. Group allocation will be revealed after the participant has completed all baseline questionnaires. To reduce burden, those allocated to multiple interventions will begin the Dexcom® G6 CGM at the baseline visit and receive the remaining interventions at baseline or within seven days.
Follow-up assessment (day 14)
All participants will repeat outcome assessments. CGM data and/or behaviour change goals will be reviewed on day 14 to identify potential opportunities to increase glucose TIR.
Final assessment visit (day 28)
Participants will be re-assessed for study outcomes and provided with handouts of resources from components not received during the intervention phase. Participants will be offered a report of their glucose sensor data and advised to consult their diabetes team with questions. Participants will receive a $20 voucher as a token of appreciation.
Safety monitoring
At the screening visit, participants who do not have a current hypoglycaemia management plan will be referred to their diabetes care team for advice. Anyone whose PHQ score is ≥ 15, DEPS-R score is ≥ 20 or self-reports recent episodes of diabetic ketoacidosis or severe hypoglycaemia at study visits will be referred to their usual diabetes care provider, general practitioner, or secondary care provider, as appropriate. Blinded CGM data will be reviewed and episodes where glucose values fall below 3.9 mmol/L overnight (10 pm to 7 am) will be reported to the participant’s diabetes care team, with their consent.
Data management
To protect confidentiality, identifiable data (e.g., name, address, date of birth, date of diagnosis) will be collected with paper questionnaires or a password protected Excel document and stored securely at each site. All other study data will be collected and managed in REDCap.
Statistical analysis
A biostatistician (J.J.H.) blinded to study group allocation will conduct all statistical analyses. Main and interaction effects will be estimated using a regression model with TIR as the outcome variable. Using effect coding (where each component is coded as 1 [received intervention] or -1 [did not receive intervention]), the main and interaction effects will be uncorrelated and therefore are similarly powered. The main effects inform the effectiveness of each component, while interaction effects inform how the components enhance or diminish the effects when together. Participants will be excluded from analyses if < 10 days of CGM data is captured and analyses using sleep data if < 3 nights of actigraphy data are obtained.
Discussion
Adolescents and young adults with type 1 diabetes are required to perform multiple self-care tasks every day. The complexities associated with adhering to intensive self-management plans highlight the need for intervention studies to target direct and indirect factors that may support young people to achieving young people’s glycaemic goals. This research uses a pragmatic approach to develop an effective, robust, and scalable intervention to support young people with type 1 diabetes to increase their glucose TIR.
The strength of this research is the factorial experimental design that enables an estimation of the main effect of each component on TIR and the interactions between components.[8] This will result in an intervention that optimises both resource-use and health outcomes because only the most active combination of components will be included and less effective components will not. Once the optimised intervention is identified then its effectiveness can be assessed in a randomised controlled trial. This research will add to the evidence of effective diabetes self-care behaviour change strategies targeting a whole-person approach to the care of young people with type 1 diabetes.
References
Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(S27):7–19.
DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(Suppl 27(S27):105–14.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–603.
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: The DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–93.
Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72.
Akhter K, Turnbull T, Simmons D. A systematic review of parent/peer-based group interventions for adolescents with type 1 diabetes: interventions based on theoretical/therapeutic frameworks. Br J Diabetes. 2018;18(2):51–65.
O’Hara MC, Hynes L, O’Donnell M, Nery N, Byrne M, Heller SR, et al. A systematic review of interventions to improve outcomes for young adults with Type 1 diabetes. Diabet Med. 2017;34(6):753–69.
Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: The multiphase optimization strategy (MOST). Springer; 2018.
Miller KM, Beck RW, Bergenstal RM, Goland RS, Haller MJ, McGill JB, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009–14.
Borus SJ, Laffel SL. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–11.
Thabit H, Prabhu JN, Mubita W, Fullwood C, Azmi S, Urwin A, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS Study. Diabetes Care. 2020;43(10):2537–43.
Boucher SE, Gray AR, Wiltshire EJ, de Bock MI, Galland BC, Tomlinson PA, et al. Effect of 6 months of flash glucose monitoring in youth with type 1 diabetes and high-risk glycemic control: a randomized controlled trial. Diabetes Care. 2020;43(10):2388–95.
Smart CE, Annan F, Higgins LA, Jelleryd E, Lopez M, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):136–54. Suppl 27(.
Alonso GT, Fink K, Maffeis C, Jannet S, Sari KV, Elizabeth D, et al. Variation in nutrition education practices in SWEET pediatric diabetes centers—an international comparison. Pediatr Diabetes. 2021;22(2):215–20.
Rose S, Boucher SE, Galland BC, Wiltshire EJ, Stanley J, Smith C, et al. Impact of high-risk glycemic control on habitual sleep patterns and sleep quality among youth (13–20 years) with type 1 diabetes mellitus compared to controls without diabetes. Pediatr Diabetes. 2021;22(5):823–31.
Barone MTU, Menna-Barreto L. Diabetes and sleep: A complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–37.
Perfect MM, Beebe D, Levine-Donnerstein D, Frye SS, Bluez GP, Quan SF. The Development of a Clinically Relevant Sleep Modification Protocol for Youth with Type 1 Diabetes. Clin Pract Pediatr Psychol. 2016;4(2):227–40.
Hadlandsmyth K, White KS, Nesin AE, Greco LA. Proposing an Acceptance and Commitment Therapy intervention to promote improved diabetes management in adolescents: A treatment conceptualization. Int J Behav Consultation Therapy. 2013;7(4):12.
Gregg JA, Callaghan GA, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: A randomized controlled trial. J Consult Clin Psychol. 2007;75(2):336–43.
Zheng K, Bruzzese JM, Smaldone A. Illness acceptance in adolescents: A concept analysis. Nurs Forum. 2019;54(4):545–52.
Barreto M, Gaynor ST. A Single-Session of Acceptance and Commitment Therapy for Health-Related Behavior Change: Protocol Description and Initial Case Examples. Behavior analysis (Washington, DC). 2019;19(1):47–59.
Hayes LL, Ciarrochi JV. The thriving adolescent: using acceptance and commitment therapy and positive psychology to help teens manage emotions, achieve goals, and build connection. Ciarrochi J, editor. Oakland: New Harbinger Publications; 2015 1 Nov.
Lewin AB, LaGreca AM, Geffken GR, Williams LB, Duke DC, Storch EA, et al. Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: the Self-Care Inventory (SCI). J Pediatr Psychol. 2009;34(9):999–1007.
Heriseanu AI, Hay P, Touyz S. The short inventory of grazing (SIG): development and validation of a new brief measure of a common eating behaviour with a compulsive dimension. J Eat Disord. 2019;7(1):4.
LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(1):257–65.
Smout M, Davies M, Burns N, Christie A. Development of the Valuing Questionnaire (VQ). J Context Behav Sci. 2014;3(3):164–72.
Johnson JG, Harris ES, Spitzer RL, Williams JBW. The Patient Health Questionnaire for Adolescents: Validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30(3):196–204.
Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33(3):495–500.
Gillanders DT, Barker E. Development and initial validation of a short form of the diabetes acceptance and Action Scale: The DAAS-Revised (DAAS-R). J Context Behav Sci. 2019;14:20–8.
Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of Short Forms From the PROMIS (TM) Sleep Disturbance and Sleep-Related Impairment Item Banks. Behav Sleep Med. 2012;10(1):6–24.
Atkinson J, Salmond C, Crampton P. NZDep2018 Index of Deprivation. Wellington; 2019.
Acknowledgements
An abstract of the OPTIMISE study protocol was accepted for presentation at the New Zealand Society for the Study of Diabetes Annual Conference, May 13–14 2022, held in Wellington, New Zealand.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
S.E.S., S.R., J.J.H., B.C.G., R.T., C.E.S., E.J.W., M.I.d.B., C.A.J. and B.J.W. were involved with the design of the study. S.E.S. and B.C.G were involved in designing the sleep extension intervention, S.E.S., M.K. and A.C., were involved in designing the values-guided self-management intervention, and S.E.S., C.E.S., S.R. and R.T. were involved in designing the snack intervention. J.J.H. planned the statistical analyses and calculated the sample size. S.R. wrote the first draft of the manuscript and all authors worked collaboratively to review and prepare the final manuscript. S.E.S. is the guarantor of this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
This protocol has been reviewed by the Ngāi Tahu Research Consultation Committee and locality-based Māori Research Committees. The study protocol was approved by the Northern A Health and Disability Ethics Committee (ethics reference: 20/NTA/127/AM03). All District Health Boards approved recruitment and conduct of the study at their site.
Consent to participate:
Written informed consent will be obtained from parents/guardians, written informed assent will be obtained from participants aged 13 to 15 years.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rose, S., Haszard, J.J., Galland, B.C. et al. The OPTIMISE study protocol: a multicentre optimisation trial comparing continuous glucose monitoring, snacking habits, sleep extension and values-guided self-care interventions to improve glucose time-in-range in young people (13–20 years) with type 1 diabetes. J Diabetes Metab Disord 21, 2023–2033 (2022). https://doi.org/10.1007/s40200-022-01089-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01089-x