Abstract
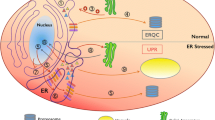
The endoplasmic reticulum (ER) is a well-characterized protein folding mechanism in eukaryotic organisms. Many secretory and membrane proteins are folded in the ER before they are translocated to their functional destination. Various conditions, such as biotic, abiotic, or physiological stresses, lead to the accumulation of unfolded and misfolded proteins in the ER, resulting in ER stress. In response to ER stress, cells initiate a protective response called the unfolded protein response (UPR) to maintain cellular homeostasis. Previous studies suggest that inositol-requiring kinase 1 (IRE1) is a universal ER stress sensor in yeast, mammals, and plants. IRE1-mediated splicing of UPR transducers, such as HAC1, XBP1, and bZIP60, triggers the UPR in yeast, mammals, and plants, respectively. In mammals, activated transcription factor 6 and double stranded RNA-activated protein kinase-like ER kinases are involved in the UPR. In plants, the additional UPR transducers bZIP28 and bZIP17 are activated by Golgi-localized S1P and S2P proteases. Subsequently, these UPR transducers are exported to the nucleus and upregulate the expression of UPR-responsive genes encoding BiP, calreticulin, calnexin, protein disulfide isomerase, and glucose-regulated protein 94 to decrease the amount of misfolded proteins and induce endoplasmic reticulum-associated degradation. In plants, the UPR signaling pathway plays an important role in ER homeostasis and normal biological processes; however, the molecular mechanisms of the UPR in plants remain poorly understood. This paper provides an overview of the regulatory and signaling mechanisms of the UPR across kingdoms. In addition, the emerging role of the UPR in plant physiology and defense response will be discussed.



Similar content being viewed by others
References
Arsham AM, Neufeld TP (2009) A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS One 4(6):e6068
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4(12):e423
Bruhat A, Averous J, Carraro V, Zhong C, Reimold AM, Kilberg MS, Fafournoux P (2002) Differences in the molecular mechanisms involved in the transcriptional activation of the CHOP and asparagine synthetase genes in response to amino acid deprivation or activation of the unfolded protein response. J Biol Chem 277(50):48107–48114
Chakrabarti A, Chen AW, Varner JD (2011) A review of the mammalian unfolded protein response. Biotechnol Bioeng 108(12):2777–2793
Chen Y, Brandizzi F (2012) AtIRE1A/AtIRE1B and AGB1 independently control two essential unfolded protein response pathways in Arabidopsis. Plant J 69(2):266–277
Chen Y, Aung K, Rolčík J, Walicki K, Friml J, Brandizzi F (2014) Inter-regulation of the unfolded protein response and auxin signaling. Plant J 77(1):97–107
Cox JS, Walter P (1996) A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87(3):391–404
Del Bem LE (2011) The evolutionary history of calreticulin and calnexin genes in green plants. Genetica 139(2):255–259
Deng Y, Humbert S, Liu J-X, Srivastava R, Rothstein SJ, Howell SH (2011) Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc Natl Acad Sci 108(17):7247–7252
Deng Y, Srivastava R, Howell SH (2013a) Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int J Mol Sci 14(4):8188–8212
Deng Y, Srivastava R, Howell SH (2013b) Protein kinase and ribonuclease domains of IRE1 confer stress tolerance, vegetative growth, and reproductive development in Arabidopsis. Proc Natl Acad Sci USA 110(48):19633–19638
Edwards D, Murray JA, Smith AG (1998) Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in Arabidopsis. Plant Physiol 117(3):1015–1022
Gebauer F, Hentze MW (2004) Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5(10):827–835
Gupta D, Tuteja N (2011) Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant Signal Behav 6(2):232–236
Gusmaroli G, Tonelli C, Mantovani R (2001) Regulation of the CCAAT-Binding NF-Y subunits in Arabidopsisthaliana. Gene 264(2):173–185
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6(5):1099–1108
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7(6):1153–1163
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633
Henriquez-Valencia C, Moreno AA, Sandoval-Ibañez O, Mitina I, Blanco-Herrera F, Cifuentes-Esquivel N, Orellana A (2015) bZIP17 and bZIP60 regulate the expression of BiP3 and other salt stress responsive genes in an UPR-independent manner in Arabidopsis thaliana. J Cell Biochem 116(8):1638–1645
Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH (2009) XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 23(19):2294–2306
Hetz C, Chevet E, Harding HP (2013) Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12(9):703–719
Humbert S, Zhong S, Deng Y, Howell SH, Rothstein SJ (2012) Alteration of the bZIP60/IRE1 pathway affects plant response to ER stress in Arabidopsis thaliana. PLoS One 7(6):e39023
Iwakoshi NN (2003) Plasma cell differentiation and the Unfolded Protein Response intersect at the transcription factor XBP-1. Nat Immunol 4:321–329
Iwata Y, Koizumi N (2005) An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc Natl Acad Sci USA 102(14):5280–5285
Iwata Y, Fedoroff NV, Koizumi N (2008) Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20(11):3107–3121
Iwata Y, Yoneda M, Yanagawa Y, Koizumi N (2009) Characteristics of the nuclear form of the Arabidopsis transcription factor AtbZIP60 during the endoplasmic reticulum stress response. Biosci Biotechnol Biochem 73(4):865–869
Iwata Y, Nishino T, Takayama S, Koizumi N (2010a) Characterization of a plant-specific gene induced by endoplasmic reticulum stress in Arabidopsis thaliana. Biosci Biotechnol Biochem 74(10):2087–2091
Iwata Y, Sakiyama M, Lee M-H, Koizumi N (2010b) Transcriptomic response of Arabidopsis thaliana to tunicamycin-induced endoplasmic reticulum stress. Plant Biotechnol 27(2):161–171
Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K (2001) Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol 3(2):158–164
Kakiuchi C, Ishiwata M, Hayashi A, Kato T (2006) XBP1 induces WFS1 through an endoplasmic reticulum stress response element-like motif in SH-SY5Y cells. J Neurochem 97(2):545–555
Kawahara T, Yanagi H, Yura T, Mori K (1997) Endoplasmic reticulum stress-induced mRNA splicing permits synthesis of transcription factor Hac1p/Ern4p that activates the unfolded protein response. Mol Biol Cell 8(10):1845–1862
Kim HS, Jung G (2014) Reactive oxygen species increase HEPN1 expression via activation of the XBP1 transcription factor. FEBS Lett 588(23):4413–4421
Kokame K, Kato H, Miyata T (2001) Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem 276(12):9199–9205
Koong AC, Chauhan V, Romero-Ramirez L (2006) Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther 5(7):756–759
Li H, Korennykh AV, Behrman SL, Walter P (2010) Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci USA 107(37):16113–16118
Li Y, Humbert S, Howell SH (2012) ZmbZIP60 mRNA is spliced in maize in response to ER stress. BMC Res Notes 5:144
Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F (2006) Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 4(3):245–254
Lisbona F, Hetz C (2009) Turning off the unfolded protein response: an interplay between the apoptosis machinery and ER stress signaling. Cell Cycle 8(11):1641–1644
Liu JX, Howell SH (2010) bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22(3):782–796
Liu Y, Li J (2014) Endoplasmic reticulum-mediated protein quality control in Arabidopsis. Front Plant Science 5:162
Liu JX, Srivastava R, Che P, Howell SH (2007a) An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19(12):4111–4119
Liu JX, Srivastava R, Che P, Howell SH (2007b) Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J 51(5):897–909
Liu JX, Srivastava R, Howell SH (2008) Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ 31(12):1735–1743
Liu Y, Burgos JS, Deng Y, Srivastava R, Howell SH, Bassham DC (2012) Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 24(11):4635–4651
Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18(6):716–731
Martínez IM, Chrispeels MJ (2003) Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell Online 15(2):561–576
Matus S, Nassif M, Glimcher LH, Hetz C (2009) XBP-1 deficiency in the nervous system reveals a homeostatic switch to activate autophagy. Autophagy 5(8):1226–1228
Merrick WC (2004) Cap-dependent and cap-independent translation in eukaryotic systems. Gene 332:1–11
Mishiba K, Nagashima Y, Suzuki E, Hayashi N, Ogata Y, Shimada Y, Koizumi N (2013) Defects in IRE1 enhance cell death and fail to degrade mRNAs encoding secretory pathway proteins in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 110(14):5713–5718
Moreno AA, Orellana A (2011) The physiological role of the unfolded protein response in plants. Biol Res 44(1):75–80
Mori K (2009) Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 146(6):743–750
Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T (2000) mRNA splicing-mediated C-terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci 97(9):4660–4665
Nagai A, Kadowaki H, Maruyama T, Takeda K, Nishitoh H, Ichijo H (2009) USP14 inhibits ER-associated degradation via interaction with IRE1α. Biochemical Biophys Rescommun 379(4):995–1000
Nagashima Y, Mishiba K, Suzuki E, Shimada Y, Iwata Y, Koizumi N (2011) Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci Rep 1:29
Nagashima Y, Iwata Y, Ashida M, Mishiba K-I, Koizumi N (2014) Exogenous salicylic acid activates two signaling arms of the unfolded Protein response in Arabidopsis. Plant Cell Physiol 55:1772–1778
Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci 104(42):16450–16455
Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13(3):349–355
Reimold AM (2001) Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300–307
Richardson CE, Kooistra T, Kim DH (2010) An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463(7284):1092–1095
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Schütze K, Harter K, Chaban C (2008) Post-translational regulation of plant bZIP factors. Trends Plant Sci 13(5):247–255
Sidrauski C, Walter P (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90(6):1031–1039
Sidrauski C, Cox JS, Walter P (1996) tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell 87(3):405–413
Srivastava R, Deng Y, Shah S, Rao AG, Howell SH (2013) BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 25(4):1416–1429
Srivastava R, Deng Y, Howell SH (2014) Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front Plant Sci 5:59
Sun L, Yang ZT, Song ZT, Wang MJ, Lu SJ, Liu JX (2013) The plant-specific transcription factor gene NAC103 is induced by bZIP60 through a new cis-regulatory element to modulate the unfolded protein response in Arabidopsis. Plant J 76(2):274–286
Sun L, Zhang S-S, Lu S-J, Liu J-X (2015) Site-1 protease cleavage site is important for the ER stress-induced activation of membrane-associated transcription factor bZIP28 in Arabidopsis. Sci China Life Sci 58(3):1–6
Tang W, Page M (2013) Transcription factor AtbZIP60 regulates expression of Ca2+-dependent protein kinase genes in transgenic cells. Mol Biol Rep 40(3):2723–2732
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258
Urade R (2009) The endoplasmic reticulum stress signaling pathways in plants. BioFactors 35(4):326–331
Wang S, Narendra S, Fedoroff N (2007) Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 104(10):3817–3822
Welihinda AA, Kaufman RJ (1996) The unfolded protein response pathway in Saccharomyces cerevisiae. Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are required for kinase activation. J Biol Chem 271(30):18181–18187
Williams B, Verchot J, Dickman MB (2014) When supply does not meet demand-ER stress and plant programmed cell death. Front Plant Sci 5:211
Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG (2002) Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA 99(25):15920–15925
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6(6):1355–1364
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281(40):30299–30304
Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107(7):881–891
Zhang P, McGrath B, Li SA, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR (2002) The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22(11):3864–3874
Zhang Y, Wang Y, Kanyuka K, Parry MA, Powers SJ, Halford NG (2008) GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2α in Arabidopsis. J Exp Bot 59(11):3131–3141
Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ (2006) The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA 103(39):14343–14348
Acknowledgments
This work was supported by grants from Next-Generation BioGreen21 Program (SSAC: PJ01109102 and PJ01106901), Rural Development Administration, Republic of Korea. RC, JHB, EYB, and MGK wrote the manuscript with input from other authors. WYK and SYL edited the paper, and gave support and conceptual advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors discussed and agreed on the contents of the paper and have no conflicts of interest to declare.
Additional information
Rupak Chakraborty and Ji Hyeong Baek have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chakraborty, R., Baek, J.H., Bae, E.Y. et al. Comparison and contrast of plant, yeast, and mammalian ER stress and UPR. Appl Biol Chem 59, 337–347 (2016). https://doi.org/10.1007/s13765-016-0167-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-016-0167-6