Abstract
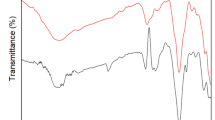
Lead (Pb) poses serious threats to plants, animals and humans once entered into the food chain via the contaminated effluents; thus, it has become imperative to remove Pb from the water stream. This study examined the potential and feasibility of compost and biogas residues for the bio-sorption (q) and removal (R) of Pb from contaminated water. To explore the objectives, a series of batch experiments was conducted at various adsorbent masses (0.5, 1.0 and 1.5 g/100 mL), Pb concentrations (30, 60 and 90 mg L−1) and contact time (15, 30, 60 and 120 min). Lead bio-sorption was decreased with increasing Pb concentration from 30 to 90 mg L−1 and sorbent biomass from 0.5 to 1.5 g/100 mL. At equilibrium state, relatively greater sorption and removal were observed from compost (q = 2.29–11.61 mg/g, R = 65–80%) as compared to biogas residues (q = 1.59–7.45 mg/g, R = 41–69%) depending upon Pb concentration and sorbent biomass in aqueous solution. Our results were better fitted with Freundlich equilibrium adsorption isotherm model (qmax = 2.882 mg/g, R2 = 0.99) and pseudo-second-order kinetics (qmax = 8.43 mg/g, R2 = 0.99). FTIR spectra indicated the presence of –OH, C–O, C=O, C=C and sp3 C–H functional groups on both adsorbents which might be involved during Pb adsorption. Compost was more porous material with well-developed cavities and had greater surface area than biogas residues (135 vs. 43 m2/g); therefore, compost has great sorption potential for heavy metal remediation from aqueous solution as compared to the biogas residues.







Similar content being viewed by others
References
Abas SNA, Ismail MHS, Kamal ML, Izhar S (2013) Adsorption process of heavy metals by low cost adsorbent. World Appl Sci J 28(11):1518–1530
Abdi O, Kazemi M (2015) A review study of biosorption of heavy metals and comparison between different biosorbents. J Mater Environ Sci 6(5):1386–1399
Ahalya N, Kanamadi RD, Ramachandra TV (2005) Biosorption of heavy metals. Electron J Biotechnol 8:258–264
Ahmad I, Akhtar MJ, Zahir ZA, Mitter B (2015) Organic amendments: effects on cereals growth and cadmium remediation. Int J Environ Sci Technol 12:2919–2928
Ahmad I, Akhtar MJ, Jadoon IBK, Imran M, Imran M, Ali S (2017) Equilibrium modeling of cadmium biosorption from aqueous solution by compost. Environ Sci Pollut Res 24:5277–5284
Ali M, Bhatia A, Kazmi AA, Ahmed N (2012) Characterization of high rate composting of vegetable market waste using Fourier transform infrared (FT–IR) and thermal studies in three different seasons. Biodegradation 23:231–242
Aziz NAA, Jayasuriya N, Fan L (2016) Adsorption study on Moringa oleifera seeds and Musa cavendish as natural water purification agents for removal of lead, nickel and cadmium from drinking water. In: IOP Conference Series: Materials Science and Engineering, eds. 1–9: Institute of Physics Publishing
Babarinde A, Ogundipe K, Sangosanya KT, Akintola BD, Hassan AOE (2016) Comparative study on the biosorption of Pb(II), Cd(II) and Zn(II) using lemon grass (Cymbopogon citratus): kinetics, isotherms and thermodynamics. Chem Int 2:89–102
Babel S, Kurniawan TA (2003) Low- cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Badmus MAO, Audu TOK, Anyata B (2007) Removal of copper from industrial wastewaters by activated carbon prepared from periwinkle shell. Korean J Chem Eng 24:246–252
Bentama J, Schmitz P, Destrac P, Espenan JM (2004) Technological innovation for the production of drinking water by membrane processes. Desalination 168(15):283–286
Bhattacharyya KG, Gupta SS (2006) Adsorption of Fe(III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption 12:185–204
Boudrahem F, Soualah A, Aissani-Benissad F (2011) Pb(II) and Cd(II) removal from aqueous solutions using activated carbon developed from coffee residue activated with phosphoric acid and zinc chloride. J Chem Eng Data 56(5):1946–1955
Chaudhary H, Ijaz M (2014) Removal of lead from wastewater by adsorption on Saponified melon peels gel. Sci Int 26(2):705–708
Dabrowski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56(2):91–106
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Edokpayi JN, Odiyo JO, Msagati TAM, Popoola EO (2015) A novel approach for removal of Lead (II) ions from wastewater using Mucilaginious leaves of Diceriocaryum Eriocarpum plant. Sustainability 7:14026–14041
Elham A, Hossein T, Mahnoosh H (2010) Removal of Zn (II) and Pb(II) ions using rice husk ih food industrial waste water. J Appl Sci Environ Manag 14:159–162
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54
Fong Lo S, Yung S, Wang M, Jer Tsai L, Lin Dong (2012) Adsorption capacity and removal efficiency of heavy metal ions by Moso and bamboo activated carbons. Chem Eng Res Des 90:1397–1406
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Ghorbani M, Eisazadeh H, Ghoreyshi A (2012) Removal of zinc ions from aqueous solution using polyaniline nanocomposite coated on rice husk. Iran J Energy Environ 3:83–88
Gupta VK, Yola ML, Atar N, Solak AO, Uzun L, Ustundağ Z (2013a) Electrochemically modified sulfisoxazole nanofilm on glassy carbon for determination of cadmium(II) in water samples. Electrochim Acta 105:149–156
Gupta VK, Yola ML, Atar N, Ustundağ Z, Solak AO (2013b) A novel sensitive Cu(II) and Cd(II) nanosensor platform: graphene oxide terminated p-aminophenyl modified glassy carbon surface. Electrochim Acta 112:541–548
Hafshejani LD, Nasab SB, Gholami RM, Moradzadeh M, Izadpanah Z, Hafshejani SB, Bhatnagar A (2015) Removal of zinc and lead from aqueous solution by nanostructured cedar leaf ash as biosorbent. J Mol Liq 211:448–456
Hanif MA, Nadeem R, Bhatti HN, Ahmad NR, Ansari TM (2007) Ni (II) biosorption by Cassia fistula (Golden shower) biomass. J Hazard Mater 139:345–355
Heraldy E, Lestari WW, Permatasaria D, Arimurtia DD (2018) Biosorbent from tomato waste and apple juice residue for lead removal. J Environ Chem Engin 6:1201–1208
Hussain AZ, Sharif KMM (2014) Removal of heavy metals from wastewater using low cost adsorbents. Arch Appl Sci Res 6(6):52–54
Ihsanullah Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161
Inglezakis VJ, Poulopoulos S (2006) Adsorption, ion exchange and catalysis: design of operations and environmental applications. Elsevier, Amsterdam
Jiang K, Sun TH, Sun LN, Li HB (2006) Adsorption characteristics of copper, lead, zinc and cadmium ions by tourmaline. J Environ Sci 18:1221–1225
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metal from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour Technol 76:63–65
Kocasoy J, Guvener Z (2009) Efficiency of compost in the removal of heavy metals from the industrial wastewater. Environ Geol 57:291–296
Kumar PS, Kirthika CVK, Kumar KS (2010) Kinetics and equilibrium studies of Pb2 + ion removal from aqueous solutions by use of nano-silversol-coated activated carbon. Braz J Chem Eng 27:339–346
Kurniawan TA, Chan GYS, Lo WH, Babel S (2006) Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98
López-Sotelo JB, Quina MJ, Gando-Ferreira L, Sanchez-Bascones M, Navas-Gracia LM (2016) Compost from poultry hatchery waste as a biosorbent for removal of Cd(II) and Pb(II) from aqueous solutions. https://doi.org/10.1002/cjce.22761
Lupean M, Bulgariu L, Macoveanu M (2012) Biosorption of Cd(II) from aqueous solution on marine green algae biomass. Environ Eng Manag J 11:607–615
Mathivanan K, Rajaram R, Annadurai G (2018) Biosorption potential of Lysinibacillus fusiformis KMNTT-10 biomass in removing lead (II) from aqueous solutions. Sep Sci Technol. https://doi.org/10.1080/01496395.2018.1442863
Meunier N, Laroulandie J, Blais JF, Dayal TR (2003) Lead removal from acidic solutions by sorption on cocoa shells: effect of some parameters. J Environ Eng 10:693–698
Milhajlovic MT, Lazarevic SS, Jankovic-Castvan IM, Kovac J, Jokic BM, Janackovic DT, Petrovic RD (2015) Kinetics, thermodynamics, and structural investigations on the removal of Pb2+, Cd2+, and Zn2+ from multicomponent solutions onto natural and Fe(III)-modified zeolites. Clean Technol Environ Policy 14:407–419
Mondal MK (2010) Removal of Pb(II) from aqueous solution by adsorption using activated tea waste. J Environ Manag 11:3266–3271
Mosa AA, El-Ghamry A, Al-Zahrani H, Selim EM, El-Khateeb A (2017) Chemically modified biochar derived from cotton stalks: characterization And assessing its potential for heavy metals removal from wastewater. Environ Biodiv Soil Securit 1:33–45
Naseem R, Tahir SS (2001) Removal of Pb(II) from aqueous solution by using bentonite as an adsorbent. Water Res 35:3982–3986
Park D, Yun YS, Moon Park J (2010) The past, present, and future trends of biosorption. Biotchnol Bioproc Eng 15:86–102
Sadaf S, Bhatti HN (2013) Evaluation of peanut husk as a novel, low cost biosorbent for the removal of Indosol Orange RSN dye from aqueous solutions: batch and fixed bed studies. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-013-0653-z
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164:1004–1011
Shah GM, Tufail N, Rashid MI, Imran M, Murtaza B, Saeed F, Farooq MAU, Waqar A, Bakhat HF (2017) Anaerobic degradation of municipal organic waste among others composting techniques improves N cycling through waste-soil-plant continuum. J Soil Sci Plant Nutri 17:529–542
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253
Wahab MA, Jellali S, Jedidi N (2010) Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Yola ML, Atar N, Qureshi MS, Ustundağ Z, Solak AO (2012) Electrochemically grafted etodolac film on glassy carbon for Pb(II) determination. Sens Actuators B Chem 171–172:1207–1215
Yola ML, Eren T, İlkimen H, Atar N, Yenikaya C (2014) A sensitive voltammetric sensor for determination of Cd(II) in human plasma. J Mole Liq 197:58–64
Acknowledgements
The study was partially funded by the COMSATS University Islamabad, Vehari-Campus, Vehari. We are equally indebted to CUI, Lahore, for the characterization of the bio-sorbents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Shah, G.M., Umm-e-aiman, Imran, M. et al. Kinetics and equilibrium study of lead bio-sorption from contaminated water by compost and biogas residues. Int. J. Environ. Sci. Technol. 16, 3839–3850 (2019). https://doi.org/10.1007/s13762-018-1865-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1865-x