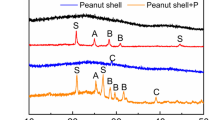
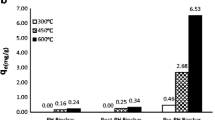
Abstract
As an alternative strategy for phosphate removal, biochar (black carbon) has characteristics superior to those of widely used adsorptive media, from both economic and environmental points of view. In this study, various types of biochar derived from oak wood, bamboo wood, maize residue, soybean stover, and peanut shell were tested for evaluation of phosphate removal. After 24 h of reaction time, the phosphate removal was limited (2.0–9.4 %) in case of general adsorptive media. However, interestingly, among various biochars, peanut shell-derived biochar (PSB) exhibited the best performance, showing the highest phosphate removal rate, 61.3 % (3.8 mg PO4−P g PSB−1). We attribute this high value to the proper structural properties of PSB, such as BET-specific surface area of 348.96 m2 g−1 and mineral/phosphorus ratio (Mg/P = 3.46 and Ca/P = 47.6). Adsorption equilibrium and kinetics of phosphate at different temperature (10, 20, and 30 °C) were well explained in the whole experimental region by Langmuir isotherm and pseudo-second-order kinetic models, respectively. The maximum adsorption capacity of PSB was 6.79 mg g−1 for phosphate at 30 °C. These findings suggest that PSB has great potential as an alternative and renewable adsorptive media for phosphate removal.





Similar content being viewed by others
References
Ahmad M, Lee SS, Dou X, Mohan D, Sung JW, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544
Ahmad M, Lee SS, Oh SE, Mohan D, Moon DH, Lee YH, Ok YS (2013) Modeling adsorption kinetics of trichloroethylene onto biochars derived from soybean stover and peanut shell wastes. Environ Sci Pollut Res 20:8364–8373
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Cao XD, Harris W (2010) Properties of dairy-manure-derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Clark T, Stephenson T, Pearce PA (1997) Phosphorus removal by chemical precipitation in a biological aerated filter. Water Res 31:2557–2563
Eberhardt TL, Min SH, Han JS (2006) Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride. Bioresour Technol 97:2371–2376
Ghaedi M, Hassanzadeh A, Kokhdan SN (2011) Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of alizarin red s and morin. J Chem Eng Data 56:2511–2520
Guan XH, Chen GH, Shang C (2007) Adsorption behavior of condensed phosphate on aluminum hydroxide. J Environ Sci 19:312–318
Hale SE, Alling V, Martinsen V, Mulder J, Breedveld GD, Cornelissen G (2013) The sorption and desorption of phosphate-P, ammonium-N and mitrate-N in cacao shell and corn cob biochars. Chemosphere 91:1612–1619
Han JS, Min SH, Kim YK (2005) Removal of phosphorous using AMD treated lignocellulosic material. For Prod J 55:48–53
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho YS, Mckay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Protect 76:332–340
Huang W, Wang S, Zhu Z, Li L, Yao X, Rudolph V, Haghseresht F (2008) Phosphate removal from wastewater using red mud. J Hazard Mater 158:35–42
Hwang MJ, Shim WG, Ryu DW, Moon H (2012) Low-pressure adsorption isotherms of aromatic compounds on polyisobutylene gel measured on a quartz crystal microbalance. J Chem Eng Data 57:701–707
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139:447–452
Kim JG, Kim JH, Moon HS, Chon CM, Ahn JS (2002) Removal capacity of water plant alum sludge for phosphorus in aqueous solutions. Chem Spec Bioavailab 14:67–73
Kim BC, Sa SH, Kim MS, Lee YK, Kim JK (2007) The limiting nutrient of eutrophication in reservoirs of Korea and the suggestion of a reinforced phosphorus standard for sewage treatment effluent. Korean Soci Water Quality 23:512–517 (In Korean)
Lee JW, Kidder M, Evans BR, Paik S, Buchanan AC III, Garten CT, Brwon RC (2010) Characterization of biochars produced from cornstovers for soil amendments. Environ Sci Technol 44:7970–7974
Lucchini P, Quilliam RS, DeLuca TH, Vamerali T, Jones DL (2014) Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ Sci Pollut Res 21:3230–3240
Mainstone CP, Parr W (2002) Phosphorus in revers-ecology and management. Sci Total Environ 282–283:25–47
Mainstone CP, Ashley S, Gunby A, Parr W, Woodrow D, Turton P, McAllem Y (1995) Development and testing of general quality assessment schemes: nutrients in rivers and canals. NRA R&D Project Record 469/11/HO. National Rivers Authority, Bristol
Mezenner NY, Bensmaili A (2009) Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem Eng J 147:87–96
Mohan D, Sarswat A, Ok YS, Charles U, Pittman J (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 193–194:122–130
Namasivayam C, Sangeetha D (2004) Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J Colloid Interface Sci 280:359–365
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Tung KL (2013) Feasibility of iron loaded ‘okara’ for biosorption of phosphorous in aqueous solutions. Bioresour Technol 150:42–49
Riahi K, Thayer BB, Mammou AB, Ammar AB, Jaafoura MH (2009) Biosorption characteristics of phosphates from aqueous solution onto Phoenix dactylifera L. date palm fibers. J Hazard Mater 170:511–519
Roberts KG, Gloy BA, Joseph S, Scott NR, Lehmann J (2010) Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol 44:827–833
Singh BP, Menchavez R, Takai C, Fuji M, Takahashi M (2005) Characterization of concentrated colloidal ceramics suspension: a new approach. J Colloid Interface Sci 300:163–168
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollution Res 10:126–139
Sø HU, Postma D, Jakobsen R, Larsen F (2011) Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Ceochim Cosmochim Acta 75:2911–2923
Suzuki T, Inomata S, Sawada K (1986) Adsorption of phosphate on calcite. J Chem Soc Faraday Trans 82:1733–1743
Tutem E, Apak R, Unal C (1998) Adsorptive removal of chlorophenols from water by bituminous shale. Water Res 32:2315–2324
Vasudevan S, Lakshmi J (2012) The adsorption of phosphate by graphene from aqueous solution. RSC Adv 2:5234–5242
Vohla C, Kõiv M, Bavor HJ, Chazarenc F, Mander Ű (2011) Filter materials for phosphorus removal from wastewater in treatment wetlands—a review. Ecol Eng 37:70–89
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div. Am Soc Civ Eng 89:31–60
Westholm LJ (2006) Substrates for phosphorus removal—potential benefits for on-site wastewater treatment. Water Res 40:23–36
Wu M, Guo Q, Fu G (2013) Preparation and characteristics of medicinal activated carbon powders by CO2 activation of peanut shells. Powder Technol 247:188–196
Yang YN, Sheng GY (2003) Pesticide adsorptivity of aged particulate matter arising from crop residue burns. J Agric Food Chem 51:5047–5051
Yang Y, Zhao YQ, Babatunde AO, Wang L, Ren YX, Han Y (2006) Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep Purif Technol 51:193–200
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammanappallil P, Yang L (2011a) Removal of phosphate from aqueous solution by biochar derived from naerobically digested sugar beet tailings. J Hazard Mater 190:501–507
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammananppallil P, Yang L (2011b) Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour Technol 102:6273–6278
Yuan JH, Xua RK, Zhang H (2011) The forms of alkalies in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhang G, Hu M, He L, Fu P, Wang L, Zhou J (2013) Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod Proc 91:158–160
Acknowledgments
This work was supported by grants from the Korea Research Council of Fundamental Science and Technology and the KIST Institutional Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kyung-Won Jung and Min-Jin Hwang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jung, KW., Hwang, MJ., Ahn, KH. et al. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 12, 3363–3372 (2015). https://doi.org/10.1007/s13762-015-0766-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0766-5