Abstract
Adaptations can occur at different hierarchical levels (e.g., cells and multicellular organisms), but it can be difficult to identify the level(s) of adaptation in specific cases. A major problem is that selection at a lower level can filter up, creating the illusion of selection at a higher level. We use optimality modeling of the volvocine algae to explore the emergence of genuine group (i.e., colony-level) adaptations. We find that it is helpful to develop an explicit model for what group fitness would be in the absence of group-level relationships between traits and group fitness. We call this “counterfactual fitness,” because in many actual cases of interest there are group-level relationships. Once counterfactual fitness is modeled, the difference between effects that filter up and genuine group selection is explicit and so, therefore, is the distinction between apparent and genuine group adaptations. We call the latter group-specific adaptations. Recognizing group-specific adaptations is important because only group-specific adaptations would cause the lower-level units (cells in this case) to be maladapted if they were to leave the group and enter a global cell-level population. Thus, as group-specific adaptations evolve, they create selective pressure for increased cohesiveness and individuality of groups. This article suggests that group-specific adaptations could be present in the simplest, earliest branching colonial volvocine species, which do not have distinct specialized cells. The article also makes predictions about the kind of empirical evidence needed to support or refute the hypothesis that a particular trait is a group-level adaptation.



Similar content being viewed by others
References
Andrewartha H, Birch C (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Bachmann T, Wagner H, Tropea C (2012) Inner vane fringes of barn owl feathers reconsidered: morphometric data and functional aspects. J Anat 221:1–8
Bouchard F (2011) Darwinism without populations: a more inclusive understanding of the “survival of the fittest.” Stud Hist Philos Biol Biomed Sci 42:106–114
Charlesworth B (1980) Evolution in age-structured populations. Cambridge University Press, Cambridge
Chen K, Liu Q, Liao G, Yang Y, Ren L, Yang H, Chen X (2012) The sound suppression characteristics of wing feather of owl (Bubo bubo). J Bionic Eng 9:192–199
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London
Donnan L, John PC (1983) Cell cycle control by timer and sizer in Chlamydomonas. Nature 304:630–633
Earnshaw-Whyte E (2012) Increasingly radical claims about heredity and fitness. Philos Sci 79:396–412
Ettl H (1983) Chlorophyta I-Phytomonadina. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer, Stuttgart, pp 1–807
Frank SA (2012) Natural selection. IV. The Price equation. J Evol Biol 25:1002–1019
Gardner A, Grafen A (2009) Capturing the superorganism: a formal theory of group adaptation. J Evol Biol 22:659–671
Godfrey-Smith P (2007) Conditions for evolution by natural selection. J Philos 104:489–516
Godfrey-Smith P (2008) Varieties of population structure and the levels of selection. Br J Philos Sci 59:25–50
Griesemer J (2000) The units of evolutionary transition. Selection 1:67–80
Hamilton W (1975) Innate social aptitudes of man. In: Fox R (ed) Biosocial anthropology. Wiley, New York
Heisler L, Damuth J (1987) A method for analyzing selection in hierarchically structured populations. Am Nat 130:582–602
Herron MD, Michod RE (2008) Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye. Evolution 62:436–451
Iyengar MOP, Desikachary TV (1981) Volvocales. Indian Council of Agricultural Research, New Delhi
Kirk D (1998) Volvox: a search for the molecular and genetic origins of multicellularity and cellular differentiation. Cambridge University Press, Cambridge
Kirk D (2005) A twelve-step program for evolving multicellularity and a division of labor. BioEssays 27:299–310
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Lyttle TW (1991) Segregation distorters. Annu Rev Genet 25:511–557
Matsumura K, Yagi T, Hattori A, Soloviev M, Yasuda K (2010) Using single cell cultivation system for on-chip monitoring of the interdivision timer in Chlamydomonas reinhardtii cell cycle. J Nanobiotechnol 8:23
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. Freeman, Oxford
Michod RE (1996) Cooperation and conflict in the evolution of individuality. II. Conflict mediation. Proc R Soc B 263:813–822
Michod RE (1999) Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton University Press, Princeton
Michod RE (2003) Cooperation and conflict mediation during the origin of multicellularity. In: Hammerstein P (ed) Genetic and cultural evolution of cooperation. MIT Press, Cambridge, pp 291–307
Nozaki H, Itoh M, Watanabe MM, Kuroiwa T (2011) Ultrastructure of the vegetative colonies and systematic position of Basichlamys (Volvocales, Chlorophyta). Eur J Phycol 31:67–72
Okasha S (2004) Multilevel selection and the partitioning of covariance: a comparison of three approaches. Evolution 58:486–494
Okasha S (2006) Evolution and the levels of selection. Oxford University Press, Oxford
Okasha S, Paternotte C (2012) Group adaptation, formal Darwinism and contextual analysis. J Evol Biol 25:1127–1139
Oldenhof H, Zachleder V, Van Den Ende H (2006) Blue- and red-light regulation of the cell cycle in Chlamydomonas reinhardtii (Chlorophyta). Eur J Phycol 41:313–320
Paley W (1802) Natural theology: or, evidence of the existence and attributes of the Deity. Taylor and Wilks, London
Parker G, Maynard Smith J (1990) Optimality theory in evolutionary biology. Nature 348:27–33
Price G (1970) Selection and covariance. Nature 227:520–521
Price G (1972) Extension of covariance selection mathematics. Ann Hum Genet 35:485–490
Roff DA (2001) Life history evolution. Sinauer, Sunderland
Roze D, Michod RE (2001) Mutation, multilevel selection, and the evolution of propagule size during the origin of multicellularity. Am Nat 158:638–654
Sober E (1993) The nature of selection: evolutionary theory in philosophical focus. University Of Chicago Press, Chicago
Sober E (2011) Realism, conventionalism, and causal decomposition in units of selection: reflections on Samir Okasha’s evolution and the levels of selection. Philos Phenom Res 82:221–231
Sober E, Wilson DS (2011) Adaptation and natural selection revisited. J Evol Biol 24:462–468
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Van Boven M, Weissing FJ (1999) Segregation distortion in a deme-structured population: opposing demands of gene, individual and group selection. J Evol Biol 12:80–93
Van Valen L (2009) How ubiquitous is adaptation? A critique of the epiphenomenist program. Biol Philos 24:267–280
Vítová M, Bišová K, Umysová D et al (2011) Chlamydomonas reinhardtii: duration of its cell cycle and phases at growth rates affected by light intensity. Planta 233:75–86
Voigt J, Münzer P (1987) The Chlamydomonas cell cycle is regulated by a light/dark-responsive cell-cycle switch. Planta 172:463–472
Wade M (1985) Soft selection, hard selection, kin selection, and group selection. Am Nat 125:61–73
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton
Wilson DS (1975) A theory of group selection. Proc Natl Acad Sci USA 72:143–146
Acknowledgments
This paper grew out of a workshop, “Stabeco: the stability of ecosystems, from ecology to environmental ethics,” funded by the “ecological engineering” program of the Ecology-Environment Institute (INEE) of the French CNRS. We thank P. Huneman for the invitation to the workshop and for editing this work along with M. van Baalen. We also thank J. Damuth, Z. Grochau-Wright, and C. Simpson for comments and M. Leslie and J. Watkins for helpful discussion. This research was supported in part by the National Science Foundation under Grants No. PHY11-25915 to the Kavli Institute for Theoretical Physics and DEB-0742383 to R. Michod. This work was also partially supported by National Aeronautics and Space Administration Grant No. NNX13AH41G to R. Michod.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Cell-Level Fitness, Scenario A
We assume that the generation time (T t , the total time between rounds of divisions) is simply 24 h. This is consistent with behavior of C. reinhardtii cells in some culture conditions. The rate of mortality of cells (M A L ) is assumed to depend on the available resources as well as on the amount of cell’s investment of limited resources in machinery for growth:
Here k m is the mortality rate that would occur if the cell invested all limiting resources in growth (as opposed to defense), and g is the proportion of limiting resources the cell invests in growth rather than defense. We assume that the actual growth rate of a cell is given by:
where k g is the ideal cell growth rate (i.e., the cell growth rate that would occur in the given conditions if the cell invested completely in the growth machinery). The amount of growth that happens in one generation is offset by the number of divisions such that:
Here, c 0 and c 1 are the initial cell sizes of the parent and offspring, respectively; k a is the actual rate of cell growth (as defined above); t s is the amount of time that a cell was able to grow (roughly corresponding to the sunlight hours); and n 0 is the number of rounds of divisions per cycle that the parent undergoes. Because n must be an integer, the initial offspring cell size is not necessarily the same as the parent initial cell size. In C. reinhardtii, the rounds of cell divisions continue the maximum number of times so as to result in offspring that are larger than some minimum threshold size, c min . The smallest possible offspring size is c min and the largest possible offspring size is just under 2c min (because cells that are at least 2c min undergo another round of divisions). The number of rounds of divisions, n, is the largest previous integer (the floor) of the number of divisions that would have resulted in exactly c min for an offspring size:
In this model, the cell is converting continuous improvement in growing time or growth rate into discrete improvements in reproduction (n) by allowing for variation in the starting size of a cell. From rearranging Eq. 12, the initial size of a parental cell in a particular generation (with i indexing the generation under consideration) is:
That is, the starting size of a cell is determined in part by the starting size of its parent as well as the number of times its parent divided. Similarly, the generation-specific value for n is:
That is, the number of times a cell divides depends on that cell’s starting size (which in turn depends in part on the number of times its parent divided, as shown in Eq. 14). When these recursive equations are considered (not shown), we can see that for fixed environmental conditions, the intergenerational variation in n depends on g, the cell’s investment in growth. If growth investment is such that the total growth tends to be near a threshold for a lower or higher n, then we see cycles of varying n values. The genotype can cycle between a lower and higher value of n on the scale of a few generations under fixed environmental conditions. Alternately, if growth investment is such that the total growth tends not to be near a threshold for a lower or higher n, then the initial cell size (c) and n appear to be stable (at least on the scale of ~50 generations). In this parameter range, a genotype that invests slightly more in growth only has the effect of making equilibrium starting cell size higher. In this model, differences in starting cell size are assumed to not affect fitness, so this effect could create a jagged relationship between g and fitness (r).
In order to avoid a jagged relationship between g and fitness, we treated c as a constant and allowed n to vary continuously. The initial size of C. reinhardtii cell is known to be very similar for daughter cells originating from mother cells with different final sizes (Donnan and John 1983), so treating c as a constant is reasonable. While non-integer values of n are certainly unrealistic, the smoother fitness curve that results from this is probably more realistic. The actual relationship between growth investment and fitness in C. reinhardtii is not critical for the points developed here. Certainly, there are many more details concerning the way in which cell cycle progression is regulated (with downstream effects on n) that are relevant for understanding the status of n value as a potential group adaptation in volvocine algae. We do not consider these volvocine-specific issues here, instead developing a simple model for the purpose of illustrating and supplementing the discussion presented in the main text.
We model the fecundity of a cell as:
Substituting from Eq. 13 without the “Floor” function and with a constant starting cell size gives:
Combining the fitness components, we get:
Colony-Level Fitness, Scenario B
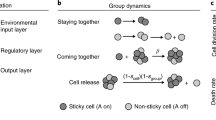
The colony-level generation time in scenario B is the same as the cell-level generation time (24 h). We have assumed a life cycle in which the generations of the higher and lower level are synchronized (Fig. 1). Whereas if a cell survives, it produces 2n offspring cells, the same is not true of colonies. Because in scenario B we assume that the colonies do not have effects that are relevant to survival and reproduction, cells within a colony survive or die independently of one another. The number of cells alive at the time of reproduction determines the number of offspring colonies a colony will have. We define x to be the probability that a cell survives one generation:
Equation 19 follows from the assumption of constant cell mortality rate (k m g). Colonies that have only one surviving cell have only one offspring colony, and so on up to colonies with the maximum number of surviving cells (2n, where n is defined as before). The overall expected number of offspring colonies for a parent colony is:
Formula 20 is the mean of a binomial distribution, which is also given by:
The colony-level fecundity is the expected number of offspring colonies, given colony survival (i.e., given that not all the cells in a colony died). Thus, the colony-level fecundity is:
We assume that the rate of colony mortality is constant, so the probability that a colony survives for one generation is exp(−M B H T t ). The probability that a colony survives for one generation is also simply the probability that not all of the cells within that colony die in one generation, or \( 1 - (1 - x^{B} )^{{2^{n} }} , \) where x B is defined as in Eq. 19. This gives:
Rearranging:
Combining the fitness components described above according to Eq. 2 gives the colony-level fitness (intrinsic rate of population increase):
Simplifying:
Substituting for x B:
Simplifying further:
In scenario B, we can see explicitly how consideration of the births and deaths of colonies gives us exactly the same bottom line (fitness) as consideration of the births and deaths of lone cells.
Cell-Level Fitness, Scenario C
In scenario C, the only way the cells differ from scenario A is their mortality rate. By substituting the scenario C mortality rate into the scenario A fitness equation we get:
Colony-Level Fitness, Scenario C
The colony-level generation time remains unchanged from the generation time in previous scenarios. To calculate the colony-level fecundity in scenario C, we begin by defining the probability that a cell will survive for one generation (x C):
The rest of the logic for calculating colony-level fecundity remains the same as in scenario B. This gives us:
The colony-level mortality in scenario C is the same as in scenario B (Eq. 24) except with the scenario B cell mortality rate replaced by the scenario C cell mortality rate:
The colony-level fitness in scenario C is the same as in scenario B except with the scenario B cell mortality rate replaced by the scenario C cell mortality rate:
The same steps (substituting and simplifying) as in scenario B can be followed to show:
Rights and permissions
About this article
Cite this article
Shelton, D.E., Michod, R.E. Group Selection and Group Adaptation During a Major Evolutionary Transition: Insights from the Evolution of Multicellularity in the Volvocine Algae. Biol Theory 9, 452–469 (2014). https://doi.org/10.1007/s13752-014-0159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-014-0159-x