Abstract
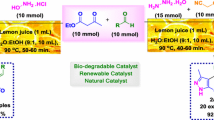
Verjuice (unripe grape juice), a natural mixture of organic acids, which is identified by pH-metric and TGA analysis, is efficiently used for the promotion of the synthesis of 5-arylmethylenepyrimidine-2,4,6-triones, via Knovenagel condensation reaction between barbituric or thiobarbituric acid and aldehydes. Verjuice is also employed for the effective synthesis of pyrano[2,3-d]pyrimidinone derivatives via a three-component reaction of barbituric acid or its thio analogue, aldehydes and malononitrile. In the same way, pyrimido[4,5-d]pyrimidinone derivatives are simply produced via the reaction of barbituric acid, aldehydes and urea or thiourea in the presence of verjuice. This green methodology rewards notable advantages including simple procedures, acceptable reaction times, easy work-up, high yields, circumventing the use of any expensive starting materials, volatile and hazardous organic solvents during the reaction and work-up process, and use of a natural, low-cost, reusable, and bio-degradable catalyst.








Similar content being viewed by others
References
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998), p. 307
P.T. Anastas, M.M. Kirchhoff, Acc. Chem. Res. 35, 686 (2002)
B.C. Ranu, K. Chattopadhyay, in Eco-Friendly Synthesis of Fine Chemicals, ch. 5, ed. by B.R. Ballini (Royal Society of Chemistry, Cambridge, 2009)
R.A. Sheldon, Green Chem. 7, 267 (2005)
C. Ameta, K.L. Ameta, Water: A Benign Solvent for the Synthesis of Various Organic Moieties (Springer, New Delhi, 2014), p. 231
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559 (2016)
M.B. Deshmukh, S.S. Patil, S.D. Jadhav, P.B. Pawar, Synth. Commun. 42, 1177 (2012)
S. Patil, S.D. Jadhav, M.B. Deshmuk, Arch. Appl. Sci. Res. 3, 203 (2011)
H. Sachdeva, R. Saroj, S. Khaturia, D. Dwivedi, Org. Chem. Int., ID 659107 (2013)
S. Patil, S.D. Jhadav, U.P. Patil, Arch. Appl. Sci. Res. 4, 1074 (2012)
R. Pal, S. Khasnobis, T. Sarkar, Chem. J. 3, 7 (2013)
J. Petronijević, Z. Bugarčić, G.A. Bogdanović, S. Stefanovićc, N. Janković, Green Chem. 19, 707 (2017)
S. Patil, S.D. Jadhav, S. Mane, Int. J. Org. Chem. 1, 125 (2011)
R. Pal, Int. J. Chem. Tech. Appl. 2, 26 (2013)
A.M. Fonseca, F.J. Monte, M.C.F. Oliveira, M.C.M. Mattos, G.A. Cordell, R. Braz-Filho, T.L.G. Lemos, J. Mol. Catal. B Enzyme 57, 78 (2009)
A. Maleki, M. Ghassemi, R. Firouzi-Haji, Pure Appl. Chem. 90, 387 (2018)
A. Maleki, R. Paydar, React. Funct. Polym. 109, 120 (2016)
A. Maleki, P. Zand, Z. Mohseni, Chem. Select 2, 2740 (2017)
A. Maleki, P. Ravaghi, M. Aghaei, H. Movahed, Res. Chem. Intermed. 43, 5485 (2017)
A. Maleki, A.A. Jafari, S. Yousefi, J. Iran. Chem. Soc. 14, 1801 (2017)
A. Maleki, R. Firouzi-Haji, Z. Hajizadeh, Int. J. Biol. Macromol. 116, 320 (2018)
M.S.P. Nickfardjam, Mitteulingen Klosterneuburg 58, 28 (2008)
I. Hayoglu, O. Kola, C. Kaya, S. öZer, H. Turkoglu, J. Food Process Preserv. 33, 252 (2009)
M. Alipour, P. Davoudi, Z. Davoudi, J. Med. Plant Res. 6, 5677 (2012)
B. Aminian, S.-M. Massoompour, A. Sadeghalvaad, G.H. Omrani, Arch. Iran Med. 6, 32 (2003)
A. Sabir, E. Kafkas, S. Tangolar, Spanish J. Agric. Res. 8, 425 (2010)
I. Jančářová, L. Jančář, A. Náplavová, V. Kubáň, Cent. Eur. J. Chem. 11, 1575 (2013)
A. Shaabani, A. Maleki, Chem. Pap. 61, 333 (2007)
A. Shaabani, M. Seyyedhamzeh, A. Maleki, F. Rezazadeh, M. Behnam, J. Comb. Chem. 11, 375 (2009)
A. Maleki, J. Rahimi, O.M. Demchuk, A.Z. Wilczewska, R. Jasiński, Ultrason. Sonochem. 43, 262 (2018)
J.D. Figueroa-Villar, C.E. Rangel, L.N. Dos Santos, Synth. Commun. 22, 1159 (1992)
K. Tanaka, X. Cheng, F. Yoneda, Tetrahedron 44, 3241 (1988)
Y. Frangin, C. Guimbal, F. Wissocq, H. Zamarlik, Synthesis 12, 1046 (1986)
S. Furuya, T. Ohtaki, Eur. Pat. Appl., EP 608565, Chem. Abstr. 121, 205395 (1994)
W.J. Coates, Eur. Pat., 351058, Chem. Abstr. 113, 40711 (1990)
N. Kitamura, A. Onishi, Eur. Pat., 163599, Chem. Abstr. 104, 186439 (1984)
G. Levitt, US Pat., 4339267, Chem. Abstr. 98, 215602 (1983)
J. Davoll, J. Clarke, E.F. Elslager, J. Med. Chem. 15, 837 (1972)
E.M. Griva, S. Lee, C.W. Siyal, D.S. Duch, C.A. Nichol, J. Med. Chem. 23, 327 (1980)
F. Shirini, M.S.N. Langarudi, O.G. Jolodar, Dyes Pigment. 123, 186 (2015)
M.M. Hanna, Eur. J. Med. Chem. 55, 12 (2012)
J.P. de la Cruz, T. Carrasco, G. Ortega, F. Sanchez de la Cuesta, Lipids 27, 192 (1992)
P. Sharma, N. Rane, V.K. Gurram, Bioorg. Med. Chem. Lett. 14, 4185 (2004)
R.B. Lichtner, G. Hutchinson, K. Hellmann, Eur. J. Cancer Clin. Oncol. 25, 945 (1989)
Y.S. Sanghvi, S.B. Larson, S.S. Matsumoto, L.D. Nord, D.F. Smee, R.C. Willis, T.L. Avery, R.K. Robins, G.R. Revankar, J. Med. Chem. 32, 629 (1989)
J.M. Khurana, K. Vij, Catal. Lett. 138, 104 (2010)
S. Kamble, G. Rashinkar, A. Kumbhar, K. Mote, R. Salunkhe, Arch. Appl. Sci. Res. 2, 217 (2010)
B.F. Mirjalili, A. Bamoniri, S.M. Nezamalhosseini, J. Nanostruct. 5, 367 (2015)
S.B. Rathod, A.B. Gambhire, B.R. Arbad, M.K. Lande, Bull. Korean Chem. Soc. 31, 339 (2010)
Y. Hu, Z. Chen, Z. Le, Q. Zheng, Synth. Commun. 34, 4521 (2004)
J.T. Li, H.G. Dai, D. Liu, T.S. Li, Synth. Commun. 36, 789 (2006)
C. Wang, J.J. Ma, X. Zhou, X.H. Zang, Z. Wang, Y.J. Gao, P.L. Cui, Synth. Commun. 35, 2759 (2005)
L.S. Gadekar, M.K. Lande, Org. Chem. Ind. J. 8, 386 (2012)
N. Daneshvar, F. Shirini, M.S.N. Langarudi, R. Karimi-Chayjani, Bioorg. Chem. 77, 68 (2018)
Z. Ren, W. Cao, W. Tong, X. Jing, Synth. Commun. 32, 1947 (2002)
N. Seyyedi, F. Shirini, M.S.N. Langarudi, RSC Adv. 6, 44630 (2016)
F. Shirini, M.S.N. Langarudi, M. Seddighi, O.G. Jolodar, Res. Chem. Intermed. 41, 8483 (2015)
A. Maleki, A.A. Jafari, S. Yousefi, Carbohydr. Polym. 175, 409 (2017)
O. Goli-Jolodar, F. Shirini, M. Seddighi, J. Iran. Chem. Soc. 13, 457 (2015)
B. Sadeghi, M. Bouslik, M.R. Shishehbore, J. Iran. Chem. Soc. 12, 1801 (2015)
D.N. Chavan, D.R. Patil, D.R. Kumbhar, M.B. Deshmukh, Chem. Sci. Rev. Lett. 4, 1051 (2015)
D.K. Yadav, M.A. Quraishi, J. Mater. Environ. Sci. 5, 1075 (2014)
J. Azizian, A. Shameli, S. Balalaie, M.M. Ghanbari, S. Zomorodbakhsh, M. Entezari, S. Bagheri, G. Fakhrpour, Orient. J. Chem. 28, 327 (2012)
M.M. Heravi, A. Ghods, K. Bakhtiari, F. Derikvand, Synth. Commun. 40, 1927 (2010)
M. Bararjanian, S. Balalaie, B. Movassagh, A.M. Amani, J. Iran. Chem. Soc. 6, 436 (2009)
G.M. Ziarani, S. Faramarzi, S. Asadi, A. Badiei, R. Bazl, M. Amanlou, Daru J. Pharm. Sci. 21, 3 (2013)
A. Mobinikhaledi, M.A. Bodaghi-Fard, Acta Chim. Slov. 57, 931 (2010)
A. Mobinikhaledi, N. Foroughifar, M.A. Bodaghi Fard, Synth. React. Inorg. Met. Org. Nano Metal Chem. 40, 179 (2010)
M.A. Bodaghifard, M. Solimannenejad, S. Asadbegi, S. Dolatabadifarahni, Res. Chem. Intermed. 42, 1165 (2016)
B. Sabour, M. Peyrovi, M. Hajimohammadi, Res. Chem. Intermed. 41, 1343 (2015)
S.V. Shinde, W.N. Jadhav, N.N. Karde, R.H. Tale, A. Chaudhari, Bull. Catal. Soc. India 8, 157 (2009)
S. Balalaie, S. Abdolmohammadi, H.R. Bijanzadeh, A.M. Amani, Mol. Divers. 12, 85 (2008)
A.H. Kategaonkar, S.A. Sadaphal, K.F. Shelke, B.B. Shingate, M.S. Shingare, Ukr. Bioorg. Acta 1, 3 (2009)
M.A.A. Mohamed, N.F.H. Mahmoud, A.M.M. El-Saghier, Chem. J. 2, 64 (2012)
M. Sh. Rostamizadeh, R. Nojavan, M. Aryan, Azad, Catal. Lett. 144, 1772 (2014)
F. Shirini, M. Mamaghani, S.V. Atghia, Catal. Commun. 12, 1088 (2011)
F. Shirini, M. Mamaghani, S.V. Atghia, Appl. Clay Sci. 58, 67 (2012)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, J. Mol. Catal. A Chem. 363, 10 (2012)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, A.R. Aliakbar, C. R. Chimie 17, 164 (2014)
F. Shirini, M. Mamaghani, M. Seddighi, RSC Adv. 4, 50631 (2014)
F. Shirini, M. Mamaghani, M. Seddighi, C. R. Chimie 18, 573 (2015)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
N.R. Dighore, P.L. Anandgaonker, S.T. Gaikwad, A.S. Rajbhoj, Res. J. Chem. Sci. 4, 93 (2014)
J.K. Rajput, J. Kaur, Chin. J. Catal. 34, 1697 (2013)
J. Albadi, A. Mansournezhad, T. Sadeghi, Res. Chem. Intermed. 41, 8317 (2015)
O. Goli-Jolodar, F. Shirini, M. Seddighi, J. Iran. Chem. Soc. 13, 475 (2016)
M. Kidwai, K. Singhal, S.Z. Kukreja, Naturforsch. 62, 732 (2007)
S.V. Shinde, W.N. Jadhav, N.N. Karade, Orient. J. Chem. 26, 307 (2010)
Acknowledgements
The authors are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safari, N., Shirini, F. & Tajik, H. Verjuice as a green and bio-degradable solvent/catalyst for facile and eco-friendly synthesis of 5-arylmethylenepyrimidine-2,4,6-trione, pyrano[2,3-d]pyrimidinone and pyrimido[4,5-d]pyrimidinone derivatives. J IRAN CHEM SOC 16, 887–897 (2019). https://doi.org/10.1007/s13738-018-1565-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1565-y