Abstract
Key message
Foliar phosphorus (P) resorption in Quercus variabilis Blume was significantly lower at a P-rich than at a P-deficient site. Moreover, P resorption strongly decreased, and nitrogen:phosphorus and carbon:phosphorus resorption ratios increased with soil P content. This demonstrates a strong link between foliar P resorption and P content in soils, and emphasizes the importance of P resorption in leaves of trees growing in soils with contrasted P content.
Context
Subtropical ecosystems are generally characterized by P-deficient soils. However, P-rich soils develop in phosphate rock areas.
Aims
We compared the patterns of nutrient resorption, in terms of ecological stoichiometry, for two sites naturally varying in soil P content.
Methods
The resorption efficiency (percentage of a nutrient recovered from senescing leaves) and proficiency (level to which nutrient concentration is reduced in senesced leaves) of 12 elements were determined in two oak (Q. variabilis) populations growing at a P-rich or a P-deficient site in subtropical China.
Results
P resorption efficiency dominated the intraspecific variation in nutrient resorption between the two sites. Q. variabilis exhibited a low P resorption at the P-rich site and a high P resorption at the P-deficient site. Both P resorption efficiency and proficiency strongly decreased with soil P content only and were positively related to the N:P and C:P ratios in green and senesced leaves. Moreover, resorption efficiency ratios of both N:P and C:P were positively associated with soil P.
Conclusion
These results revealed a strong link between P resorption and P stoichiometry in response to a P deficiency in the soil, and a single- and limiting-element control pattern of P resorption. Hence, these results provide new insights into the role of P resorption in plant adaptations to geologic variations of P in the subtropics.
Similar content being viewed by others
1 Introduction
Nutrient resorption comprises a process of nutrient remobilization from senesced organs to living tissues within a plant, which serves as a nutrient conservation strategy that plays an essential role in the adaptation of plants to environments with variable nutrient concentrations (Killingbeck 1986; May and Killingbeck 1992; Castle and Neff 2009). Nutrient resorption has been measured as resorption efficiency or resorption proficiency. Resorption efficiency refers to the percentage of a nutrient that is withdrawn from senesced organs prior to their abscission (Aerts 1996), while resorption proficiency is the level to which nutrient concentration is reduced in senesced organs (or the nutrient concentration levels within senesced organs, where low nutrient concentrations in senesced organs indicate a high nutrient proficiency) (Killingbeck 1996). Both measures have continued to receive widespread attention in many studies (McGroddy et al. 2004; Rentería et al. 2005; Richardson et al. 2005; Drenovsky and Richards 2006; Rentería and Jaramillo 2011; Yuan and Chen 2015). Plants with higher nutrient resorption exhibit an enhanced capacity to reduce the dependence of plants on the status of soil nutrients (Killingbeck 1996; Yuan and Chen 2009a), while increasing nutrient use efficiency (Yuan et al. 2006). Typically, ~ 50% of leaf phosphorus (P) may be recycled through the resorption process (Yuan and Chen 2009a; Vergutz et al. 2012); hence, it is likely that nutrient resorption is particularly important to plants in subtropical and tropical regions, which are subject to P deficiency and limitation (Vitousek et al. 2010).
Although higher levels of nutrient resorption in plants grown in infertile soils are to be expected, no consistent pattern was detected with respect to nutrient availability in the soils. The relationship of nutrient resorption with soil nutrient status was mostly negative, i.e., increasing nutrient resorption with decreasing nutrient availability (Aerts and De Caluwe 1994; Yuan and Chen 2015); however, positive (Nambiar and Fife 1987; Sabaté et al. 1995) and a lack of a clear relationship (Wright and Westoby 2003) have also been reported. Most of these studies had their focus set primarily on single-element control patterns of nutrient resorption, e.g., P resorption efficiency (PRE) in relation to P availability, or nitrogen (N) resorption efficiency (NRE) in relation to N availability separately (Li et al. 2010; Yuan and Chen 2015). Recently, See et al. (2015) initially and successfully employed the multiple-element control (which suggested that the foliar resorption of one element could be driven by the availability of another element, based on the resource optimization theory) to explain the variable relationships between nutrient resorption and nutrient availability. They observed that P resorption was strongly affected by the N content of soil in northern hardwood forests, where productivity was limited by both N and P. Studies that examined the response of nutrient resorption in natural habitats provided valuable information on the long-term adaptive response (adaptation) to environmental changes, while nutrient addition experiments presented direct evidence for short-term responses (acclimation) to soil nutrients (Yuan and Chen 2012). Moreover, high levels of added nutrients and steep increases of added nutrients rarely occurred in natural habitats. Cao et al. (2014) suggested that the adaptation of dominant plants in phosphorus-enriched areas to local heterogeneous environments was characterized by certain genetic differentiations. Therefore, similar to Hayes et al. (2014), who showed the major influence of the strong gradient in N and P availability along a 2-million-year dune chronosequence on leaf nutrient resorption, it was necessary to study the response of nutrient resorption under naturally geological nutrient varying habitats, which might confer valuable information in understanding how plants adapt to their environments across long-time scales, particularly trees with prolonged longevity.
Subtropical soils are generally characterized by deficiencies in phosphorous (P), calcium (Ca), and magnesium (Mg), and an enrichment of iron (Fe) and aluminum (Al). In particular, P-deficient soils result in the P-limited stoichiometric traits of plants, such as low P content and high N:P and C:P ratios in the leaves (Reich and Oleksyn 2004; Han et al. 2005; Reich 2005; Sun et al. 2015). However, in some areas of subtropical China, P-deficient sites are often mixed with P-rich sites on P-rich ores, which results in significant variations of P and other elements (e.g., N, Ca, and Mg) (Yan et al. 2011; Zhou et al. 2015; Ji et al. 2017). Xiao et al. (2009) found P-rich ecotypes of Pilea sinofasciata and Polygonum hydropiper in P-rich ore areas. Plants growing in P-enriched soils had considerably higher P content and lower N:P ratios (Yan et al. 2011). Quercus variabilis showed highly variable nutrient concentrations within its leaves across the subtropical P-rich and P-deficient sites (Zhou et al. 2015). Over the last decade, many studies have investigated the stoichiometric composition (C:N, N:P, and C:P ratios) of leaves, which revealed an intimate relation with plant growth (McGroddy et al. 2004; Reich and Oleksyn 2004; Han et al. 2005; Ågren 2008). Moreover, Reed et al. (2012) used a stoichiometric approach to assess foliar resorption patterns at a variety of scales, and found that tropical sites had N:P resorption ratios of < 1, whereas plants growing on highly weathered tropical soils maintained the lowest N:P resorption ratios. Thus, similar to responses of the stoichiometric traits of live leaves to variable soil nutrients in subtropical areas, there might be intriguing stoichiometric patterns, such as N:P ratios and N:P resorption ratios, in senesced leaves and in foliar resorption in naturally geologic-P varying areas.
Deciduous oak, Q. variabilis, is a pan Eastern Asian tree species with ecological, economic, and cultural importance in China, Korea, and Japan (Chen et al. 2012). Both the resorption efficiencies and proficiencies of Q. variabilis were strongly impacted by climate and the respective concentrations of nutrients in soils and green leaves across temperate and subtropical areas (Sun et al. 2016). In the Central Yunnan Plateau, Q. variabilis stands have declined significantly due to serious historical disturbances, such as cutting, grazing, and agricultural activities, and the remaining stands are highly fragmented. Therefore, it is critical to understand the status of nutrient content and nutrient resorption of Q. variabilis and other trees in the context of silviculture, forest management, and the restoration of abandoned open-pit mines in this region (Xiao et al. 2009).
There were substantial differences in the elemental composition of leaves, acorns, and even the parasitic insects in the acorns of Q. variabilis, due to variable soil nutrients at P-rich sites on phosphate rocks and P-deficient sites on non-phosphate rocks in Central Yunnan, near Kunming, where P-rich soils develop from the natural, long-term mineralization of phosphorus rocks (Zhou et al. 2015; Ji et al. 2017). This indicated that nutrient resorption should differ in such contrasting P habitats (Brant and Chen 2015). In this study, non-minerals (C), macro-minerals (N, P, potassium (K), calcium (Ca), and magnesium (Mg)), essential micro-elements (iron (Fe), manganese (Mn), zinc (Zn), copper (Cu)), and non-essential elements (aluminum (Al) and sodium (Na)), as well as stoichiometric ratios (C:N, N:P, and C:P) were determined in live and senesced leaves. Our study aimed to reveal the patterns of nutrient resorption in naturally geologic-P varying areas in terms of ecological stoichiometry. Since long-term pedogenesis leads to important changes in the availability of soil nutrients, particularly P limitation, which has greatly influenced foliar nutrient concentrations and resorption and plant growth (Laliberté et al. 2012; Hayes et al. 2014; Turner and Laliberté 2015), we hypothesized that (1) leaf nutrient resorption of Q. variabilis between the P-rich and P-deficient sites would be significantly different, where P resorption would have the largest contribution in influencing the difference; (2) Q. variabilis would possess high P resorption at the P-deficient site, while having low P resorption at the P-rich site; (3) P resorption would significantly decrease with increasing P content in soils; and (4) there would be intimate relationships between P resorption and stoichiometry of foliar and soil P (such as N:P and C:P ratios), and also between resorption ratios of N:P and C:P and soil P content.
2 Materials and methods
2.1 Study sites
The P-rich site (developed on P-rich phosphate rocks) was located at Wenquan Village, Anning County (102° 26′ 47.58″ E, 24° 58′ 54.38″ N, 1869 m a.s.l), and the P-deficient site (developed on non-phosphate rocks) was located at Fulong Village, Mouding County (101° 32′ 35.62″ E, 25° 14′ 48.25″ N, 1846 m a.s.l), in the Yunnan Province of Southwest China. Table S1 illustrates the chemical compositions of the phosphate rocks in Anning County (Tao 2005) and non-phosphate rocks (the Matoushan Formation rocks) in Mouding County (Shi et al. 2011). The chemical characteristics of the soils at the P-rich site and the P-deficient site are shown in Table S2. The climate was similar between the two P-type sites in 2015, with a mean annual temperature of 15.5 °C and mean annual precipitation of 897.5 mm. Moreover, the mean monthly temperature and precipitation of Anning and Mouding Counties from 1981 to 2010 are shown in Fig. S1. At these sites, Q. variabilis stands are natural forests at the mature stage, pure stands, or mixtures with other tree species, including Yunnan Pine (Pinus yunnanensis French) (Wu et al. 1987).
2.2 Sampling
Fifteen canopy trees were randomly selected (distance between the selected trees was > 20 m). The size of each stand where the trees were selected was ~ 200 m × 600 m. The height and diameter at breast height (DBH) were measured. At the Wenquan site, the Q. variabilis trees ranged from 8.5 to 23 m in height, and from 22.3 to 40 cm in DBH. At the Fulong site, the Q. variabilis trees ranged from 11 to 16 m in height, and from 18.9 to 38.5 cm in DBH. Surface soil cores (0–10 cm, 3 cm Ø) were collected from five spots that were systematically arranged around each selected tree at 1.5 m from the trunk. The collected samples were mixed to form one composite soil sample for each tree. In March, the leaves began to sprout from the trees, and the peak time for leaves to fall was mid-December. Thus, soil and mature green leaf samples were collected in mid-August, 2015. Approximately, 200 g of mature, green canopy leaves (fully exposed to sunlight) were collected from each selected tree. Subsequently, ~ 200 g of newly shed and fresh senesced leaves were collected from the top layer of the forest floor in mid-December, 2015, under the canopy of each selected tree at 1 m from the trunk.
All samples were immediately transported to the laboratory. Following the removal of mineral particulates on the leaf surfaces with wet absorbent cotton; all leaves were dried at 105 °C for 30 min, and then at 60 °C for 48 h. The soil samples were air dried for 4 weeks to a constant weight. Following grinding, the dried samples were sieved through a 60-mesh sieve (0.25 mm Ø) for chemical analysis.
Fifteen leaves were randomly selected from the leaf samples collected from each tree. Leaf area was determined with a leaf area meter AM300 (ADC BioScientific Ltd., Herts, UK), and leaf dry mass was obtained after drying at 65 °C for 72 h. Leaf mass per area (LMA) was calculated as the leaf dry mass per one sided leaf area.
2.3 Chemical analysis
All soil and leaf samples were digested with trace metal-grade nitric acid and diluted with distilled water. Total C and N concentrations were determined with an elemental analysis-stable isotope-ratio mass spectrometer (Vario ELIII; Elementar, Germany). Total concentrations of P, K, Ca, Mg, Fe, Mn, Zn, Cu, Al, and Na were obtained with a plasma optical emission spectrometer (ICP-OES) (Iris Advantage 1000; Thermo Jarrell Ash, Franklin, MA). The concentrations of all elements were expressed as milligrams per gram of dry weight. All of the analyses were conducted at the Instrumental Analysis Centre at Shanghai Jiao Tong University.
2.4 Resorption efficiency
Nutrient resorption efficiency (RE, %) calculations followed (Van Heerwaarden et al. 2003; Wright and Westoby 2003; Yuan and Chen 2009a).
where RE is resorption efficiency, and Nug and Nus are the concentrations in green and senesced leaves, respectively. The constant of mass loss correction factor (MLCF) used for Q. variabilis was 0.784, according to Vergutz et al. (2012) and Sun et al. (2016).
For C, N, and P, the resorption efficiency was represented by CRE, NRE, and PRE, respectively. The resorption efficiency ratios for C:N, N:P, and C:P were calculated as loge(CRE/NRE), loge(NRE/PRE), and loge(CRE/PRE), respectively (Reed et al. 2012).
Nutrient resorption proficiency was defined as the nutrient concentration in senesced leaves; low nutrient concentration in senesced leaves corresponded to high nutrient proficiency (Killingbeck 1996).
2.5 Statistical analyses
Nutrient resorption efficiencies, concentrations, and ratios were log-transformed when required to improve normality. Student’s t test was conducted with SPSS 18.0 (SPSS Inc., USA) to test for differences in nutrient concentrations and the C:N, N:P, and C:P molar ratios of green leaves, senesced leaves, and soils, as well as variations in foliar nutrient resorption efficiencies and resorption efficiency ratios between P-rich and P-deficient sites. By using R version 3.3.2 (The R Foundation for Statistical Computing, 2016) and including all of the sample trees, generalized linear models (GLM) were employed to represent the relationship between resorption, and soil or leaf chemistry, and the relationship between LMA and nutrient concentrations or resorption, across P-rich and P-deficient sites. Foliar resorption efficiencies, nutrient concentrations and/or stoichiometric ratios in green leaves and senesced leaves and resorption efficiency ratios were utilized as dependent variables, whereas nutrient concentrations or/and stoichiometric ratios in green leaves and soils and LMA and site types were employed as independent variables. We calculated the partial R2 using the rsq. partial function in the rsq package to describe the proportion of variance explained by the independent variable (Zhang 2017). Moreover, in order to show the intraspecific variation of the leaf resorption of multi-elements in Q. variabilis between the two P-type sites, and to identify resorption of which element had largest contribution in influencing the variation, partial least squares-discriminant analysis (PLS-DA) was used. The PLS-DA was conducted with the SIMCA13.0 software package (Umetrics, Umea, Sweden) to discriminate 30 Q. variabilis samples between the P-rich and P-deficient sites, using foliar nutrient resorption efficiencies of C, N, P, K, Ca, Mg, Fe, Mn, Zn, Cu, Al, and Na. Moreover, significant differences between the PLS-DA models were analyzed by CV-ANOVA (analysis of variance testing of cross-validated predictive residuals) test. A loading plot was generated, and the first principal component of variable importance projection (VIP) was obtained from the PLS-DA. Moreover, In order to select the resorption of which element had most potent and significant effect on discrimination of oak trees between the two P-type sites, VIP values that exceeded 1.0 and Student’s t test (p < 0.05) between two comparison groups were applied.
2.6 Data availability
The datasets generated and analyzed during the current study are available in the Zenodo repository.
(https://doi.org/10.5281/zenodo.1183815) (Ji and Liu 2018).
3 Results
3.1 Elemental stoichiometry of soils, green leaves, and senesced leaves
Concentrations of all the elements in soils were significantly higher at the P-rich site than at the P-deficient site with the exception of Ca. C:N, N:P, and C:P molar ratios in soil differed significantly between the two P-type sites (Table 1).
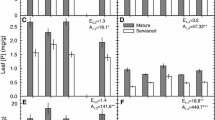
C, N, P, K, Mg, Fe, Zn, and Al concentrations of green leaves were significantly higher, but the C:N, N:P, and C:P molar ratios in green leaves were significantly lower, at the P-rich site than at the P-deficient site (Table 1).
The C, N, P, Mg, Fe, and Zn concentrations of senesced leaves were also significantly higher, but the C:N, N:P, and C:P molar ratios in senesced leaves were significantly lower, at the P-rich site than at the P-deficient site (Table 1).
3.2 Nutrient resorption efficiency and resorption efficiency ratios
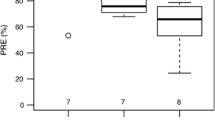
The resorption efficiencies of N, P, and Zn were smaller, and the resorption efficiency of Al was greater, at the P-rich site than those at the P-deficient site. The PRE (63%) at the P-deficient site was twice as high as the PRE (32%) at the P-rich site, whereas the NRE at the P-deficient site was only 7% higher (Table 1).
PLS-DA allows calculating synthetic variables, called latent variables, along which the scores are maximized to discriminate the groups of samples according to the studied discriminating factor, i.e., site types. A PLS-DA was performed for site type effect (Fig. 1a, b). As expected, the oak trees at the P-rich site were significantly distinct from those at the P-deficient site along the first component (Fig. 1a, CV-ANOVA p < 0.001) on PLS-DA by foliar nutrient resorption efficiencies. Examination of the first component loadings (Fig. 1b) and VIP values (Fig. 1c) showed that the major discriminant variables included the resorption efficiencies of N, P, Zn, and Al. PRE (VIP > 2) was more important than the resorption efficiencies of N, Zn, and Al (VIP > 1) in the discrimination of the sampled trees between the two P-type sites (Fig. 1c).
PLS-DA of foliar nutrient resorption efficiencies of C, N, P, K, Ca, Mg, Fe, Mn, Zn, Cu, Al, and Na of Q. variabilis at P-rich (circles) and P-deficient (triangles) sites. Score scatter plot (a) and loading scatter plot (b) from the PLS-DA model of significant components (R2X = 0.37, R2Y = 0.84, Q2 = 0.66, and CV-ANOVA: p < 0.001), and VIP value plot (c) showing the importance of the variables in discriminating sample trees between the P-rich (circles) and P-deficient (triangles) sites. Resorption efficiencies of K, Ca, Mg, Fe, Mn, Zn, Cu, Al, and Na were represented by KRE, CaRE, MgRE, FeRE, MnRE, ZnRE, CuRE, AlRE, and NaRE, respectively. The score plot indicated the two clusters of the P-rich and P-deficient groups, and the first component separated the two clusters. The loading plot indicated the variables along the first component which contributed for grouping. Here, R2 was the fraction of sum of squares of all the X’s or Y’s explained by the current component, and Q2 was the fraction of the total variation of X’s that can be predicted by a component determined by cross-validation. The ellipse indicated the Hotelling’sT2 (95%)
Moreover, the resorption efficiency ratios of C:N, N:P, and C:P at the P-rich site were 8.3, 90, and 81.8% higher than those at the P-deficient site (Table 1).
3.3 Nutrient resorption in relation to nutrient variation and stoichiometry
The PRE decreased significantly, while P concentrations in senesced leaves increased, with green leaf P and soil P. Moreover, the PRE increased, while P concentrations in senesced leaves decreased, with N:P and C:P ratios in green leaves and soils (Table 2).
However, no regular pattern was found for the CRE and NRE. The NRE increased with N concentrations in green leaves, whereas the CRE and C concentrations in senesced leaves were significantly affected by the N and C concentrations of green leaves. Moreover, the NRE decreased, while the C concentration in senesced leaves increased with C:N ratios in green leaves and soils (Table 2).
N, C, and P concentrations in senesced leaves decreased significantly with NRE, CRE, and PRE, respectively, while N:P and C:P ratios in the senesced leaves increased significantly with PRE (Table 3).
3.4 Nutrient resorption ratios in relation to nutrient variation
Partial R2 showed that both N:P and C:P resorption efficiency ratios increased only with higher concentrations of soil P and green leaf P (p < 0.001) (Table 4).
4 Discussion
4.1 P resorption at P-rich and P-deficient sites
The significantly higher PRE and NRE, particularly PRE, of Q. variabilis in soils with lower P and N were consistent with the patterns of nutrient availability on nutrient resorption generally observed (Aerts and De Caluwe 1994; Aerts and Chapin 1999; Yuan and Chen 2015). Moreover, the PRE on the P-rich site was 32%, which was almost the lowest among the values reported for deciduous tree species (Aerts 1996; Yuan and Chen 2009a), whereas the soil P concentration (6.5 mg g−1) that was the highest ever reported (Han et al. 2005). The variations in soil P resulted in significant intraspecific variations in the stoichiometric traits of Q. variabilis leaves and acorns, as well as of weevil larvae, an insect that feeds on acorns (Zhou et al. 2015; Ji et al. 2017). Therefore, the primary importance of PRE in the discrimination of Q. variabilis between the P-rich and P-deficient sites suggested that PRE was dominant in influencing the intraspecific variation in the leaf nutrient resorption of Q. variabilis between the two P-type sites (May and Killingbeck 1992).
Killingbeck (1996), McGroddy et al. (2004), Richardson et al. (2005), and Yuan and Chen (2009b) emphasized the importance of resorption proficiency (the level to which nutrient concentration is reduced in senesced organs) in assessing nutrient resorption. Among a wide range of species, the P resorption proficiency was in the range of from 0.01 to 0.21% on a mass basis, and P resorption was highly proficient in the plants with P concentrations in senesced leaves of below 0.05% (Killingbeck 1996; Rentería and Jaramillo 2011). In this study, P resorption at the P-deficient site was significantly and highly proficient, as the concentrations of P in senesced leaves was 0.055%, and the P resorption at the P-rich site was far less proficient, with the concentrations of P in the senesced leaves being 0.198% (Killingbeck 1996). As such, the patterns were consistent with those of PRE, which suggested the high strength of the P limitation in the ecosystems with P-deficient soils. Therefore, the Q. variabilis at the P-deficient site could be characterized as having implemented a highly conservative P-use strategy (Hayes et al. 2014).
The nutrient requirements of plants are generally met through uptake from soils and resorption from senescing leaves, where the balance between the use of resorption-derived and soil-derived nutrients are set by their relative costs (Aerts 1996; Wright and Westoby 2003; Yuan and Chen 2009a). Based on the theoretical model of Wright and Westoby (2003), under low fertility conditions, the energetic costs of resorbing nutrients from senescing leaves were lower than that of uptaking nutrients directly from the soil. From a cost-benefit perspective, our results suggested that the costs of resorption relative to uptake from soils were less for Q. variabilis at the P-deficient site (Scalon et al. 2017). For this study, the two P-type sites were similar in climatic conditions, but different in soil P concentrations. Thus, at the P-rich site, the energy cost of acquiring nutrients from the soil should be far lower than resorbing them from senescing leaves; thus, Q. variabilis reduced P resorption, particularly P resorption proficiency. In comparison, at the P-deficient site, the cost of nutrient uptake from soils might be more expensive than nutrient resorption from senescing leaves; therefore, Q. variabilis had a higher P resorption, especially the higher P resorption proficiency (Wright and Westoby 2003; Kobe et al. 2005; Scalon et al. 2017). Moreover, species growing in diverse nutritional conditions can develop nutrient-based ecotypes (populations) (Kawecki and Ebert 2004; Savolainen et al. 2007). Therefore, these differences in leaf nutrient resorption likely reflect the adaptation of Q. variabilis to variations of soil elements in the local environment.
4.2 P resorption in relation to geologic P variation
General patterns of N and P resorption with nutrient availability have not been observed (Aerts 1996; See et al. 2015); rather, there are evidences on the multiple-element control pattern of P resorption. For example, strong influences of soil N on P resorption occurred in northern hardwood forests, where productivity was primarily limited by N (Vadeboncoeur 2010; See et al. 2015). P resorption decreased only with the addition of N (not P) in a semi-arid steppe of Northern China, where plant productivity exhibited dominant N control (Lü et al. 2013); moreover, plant PRE were negatively impacted by the combination of N and P fertilization (Yuan and Chen 2015). In this study, although both soil N and P content at the P-rich site were significantly higher than that at the P-deficient site, there was a significantly negative correlation only between soil P content and P resorption (both PRE and P resorption proficiency), but no significant relationship between soil N content and P resorption, or between soil N content and N resorption. This may have been due to a fact that the correlation between soil N availability and the responses of P resorption usually was species-specific, with positive, negative, or neutral correlations (Kozovits et al. 2007; Lü et al. 2016). Furthermore, plant productivity in subtropical regions was always primarily limited by P availability, which revealed an ultimate P limitation (Schindler 1977; Vitousek et al. 2010). Thus, the only significant relationship between soil P and P resorption may be due to the fact that the response of plants to P variations was much more sensitive than to N variations at geologic-P varying areas (Lü et al. 2013; Lü et al. 2016). Thus, our results did not support the multiple-element control pattern of P resorption and showed evidences of the single-element control of P resorption only (e.g., strong influences of P resorption by soil P availability). Moreover, LMA had no effect on both PRE and P resorption proficiency (Table S3). These evidences suggested that P resorption may be dependent on an element (N or P) that is most and primarily limiting in ecosystems.
4.3 P resorption in relation to nutrient stoichiometry
For this study, it was observed that there were complex correlations between nutrient resorption and stoichiometric ratios. Firstly, the positive relationships between PRE and C:P and N:P ratios in senesced leaves (Table 3) suggested that the negative relationship between PRE and soil P content, and the return of organic P to soils would be affected by the indirect effect of PRE on litter decomposition (Vergutz et al. 2012) through the modification of P concentrations, C:P ratios, and N:P ratios in senesced leaves (Vitousek 1998; Aerts and Chapin 1999). Secondly, positive correlations occurred between P resorption and N:P and C:P ratios in both green and senesced leaves, as well as between NRE and C:N ratios in senesced leaves (Tables 2 and 3). The lower green leaf C:N, N:P, and C:P ratios at the P-rich site suggested that Q. variabilis had higher growth rates with high soil P (Elser et al. 2003; Makino et al. 2003), which was consistent with our observation on tree height and breast height diameter (Fig. S2). There were also negative correlations of tree height with C:P ratios in green leaves and of tree height and DBH with C:N ratios in senesced leaves (Fig. S3). Therefore, all of these results suggested that P resorption, and perhaps also NRE, were indirectly associated with Q. variabilis growth and productivity.
4.4 Resorption stoichiometry in relation to geologic-P variation
The lower resorption efficiency ratios of C:P and N:P at the P-deficient site, and the only positive association of N:P and C:P resorption ratios with foliar and soil P concentrations, also elucidated the influences of nutrient availability on resorption stoichiometry (Aerts 1996; Reed et al. 2012). Our results were consistent with the low N:P resorption ratio in tropical areas, where soil P is typically limited due to high weathering and strong leaching (Reed et al. 2012), which suggested that the N:P resorption ratio might also reflect soil P availability.
5 Conclusion
Based on the examination of the resorption efficiency and proficiency of 12 elements for Q. variabilis populations at two natural P-rich and P-deficient sites in subtropical environments, four conclusions were obtained. First, P resorption efficiency was dominant in influencing the intraspecific variation in leaf nutrient resorption of Q. variabilis between the two P-type sites, and Q. variabilis possessed low P resorption efficiency and resorption proficiency at the P-rich site, while having high P resorption efficiency and resorption proficiency at the P-deficient site, which suggested that Q. variabilis might employ an energy-efficient strategy to meet its nutrient needs. Second, both P resorption efficiency and resorption proficiency were strongly affected only by soil P, which was primarily limiting in these ecosystems, showing a single- and limiting-element control pattern of P resorption. Third, both P resorption efficiency and resorption proficiency were positively related to N:P and C:P ratios in soils, green leaves, and senesced leaves, which suggested that P resorption was indirectly associated with Q. variabilis growth and productivity in these ecosystems. Fourth, the resorption efficiency ratios of both N:P and C:P were positively associated with soil P concentrations, serving as a potential indication of soil P limitation. These results assisted in elucidating nutrient resorption patterns as they related to geological nutrient variations, toward contributing new insights into the role of P resorption in plant adaptations to variations of P in the subtropics. The results of the present study may also have significant implications for understanding how terrestrial plants respond to anthropogenic P loading into the ambient environment.
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608. https://doi.org/10.2307/2261481
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Aerts R, De Caluwe H (1994) Nitrogen use efficiency of Carex species in relation to nitrogen supply. Ecology 75:2362–2372. https://doi.org/10.2307/1940890
Ågren GI (2008) Stoichiometry and nutrition of plant growth in natural communities. Annu Rev Ecol Evol Syst 39:153–170. https://doi.org/10.1146/annurev.ecolsys.39.110707.173515
Brant AN, Chen HYH (2015) Patterns and mechanisms of nutrient resorption in plants. Crit Rev Plant Sci 34:471–486. https://doi.org/10.1080/07352689.2015.1078611
Cao J, Fu D, Yan K, Wang T, Zhang Y, Zhang Y, Zhang L, Duan C (2014) The ecological differentiation of dominant plants in phosphorus-enriched areas within the Dianchi Lake basin. Chin J Ecol 33:3230–3237. https://doi.org/10.13292/j.1000-4890.2014.0284
Castle SC, Neff JC (2009) Plant response to nutrient availability across variable bedrock geologies. Ecosystems 12:101–113. https://doi.org/10.1007/s10021-008-9210-8
Chen D, Zhang X, Kang H, Sun X, Yin S, Du H, Yamanaka N, Gapare W, Wu H, Liu C (2012) Phylogeography of Quercus variabilis based on chloroplast DNA sequence in East Asia: multiple glacial refugia and mainland-migrated island populations. PLoS One 7:e47268. https://doi.org/10.1371/journal.pone.0047268
Drenovsky RE, Richards JH (2006) Low leaf N and P resorption contributes to nutrient limitation in two desert shrubs. Plant Ecol 183:305–314. https://doi.org/10.1007/s11258-005-9041-z
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6:936–943. https://doi.org/10.1046/j.1461-0248.2003.00518.x
Han W, Fang J, Guo D, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385. https://doi.org/10.1111/j.1469-8137.2005.01530.x
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Ji H, Du B, Liu C (2017) Elemental stoichiometry and compositions of weevil larvae and two acorn hosts under natural phosphorus variation. Sci Rep 7:45810. https://doi.org/10.1038/srep45810
Ji H, Liu C (2018) Comparison of nutrient resorption stoichiometry of Quercus variabilis Blume from two contrasting geologic-phosphorus sites. V1. Zenodo. [Dataset]. https://doi.org/10.5281/zenodo.1183815
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. https://doi.org/10.1111/j.1461-0248.2004.00684.x
Killingbeck KT (1986) The terminological jungle revisited: making a case for use of the term resorption. Oikos 46:263–264. https://doi.org/10.2307/3565477
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727. https://doi.org/10.2307/2265777
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792. https://doi.org/10.1890/04-1830
Kozovits AR, Bustamante MMC, Garofalo CR, Bucci S, Franco AC, Goldstein G, Meinzer FC (2007) Nutrient resorption and patterns of litter production and decomposition in a Neotropical savanna. Funct Ecol 21:1034–1043. https://doi.org/10.1111/j.1365-2435.2007.01325.x
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll KH, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the South-Western Australia biodiversity hotspot. J Ecol 100:631–642. https://doi.org/10.1111/j.1365-2745.2012.01962.x
Lü X, Reed SC, Yu Q, Han X (2016) Nutrient resorption helps drive intra-specific coupling of foliar nitrogen and phosphorus under nutrient-enriched conditions. Plant Soil 398:111–120. https://doi.org/10.1007/s11104-015-2642-y
Lü X, Reed SC, Yu Q, He N, Wang Z, Han X (2013) Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob Chang Biol 19:2775–2784. https://doi.org/10.1111/gcb.12235
Li X, Zheng X, Han S, Zheng J, Li T (2010) Effects of nitrogen additions on nitrogen resorption and use efficiencies and foliar litterfall of six tree species in a mixed birch and poplar forest, northeastern China. Can J For Res 40:2256–2261. https://doi.org/10.1139/X10-167
Makino W, Cotner JB, Sterner RW, Elser JJ (2003) Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct Ecol 17:121–130. https://doi.org/10.1046/j.1365-2435.2003.00712.x
May JD, Killingbeck KT (1992) Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics. Ecology 73:1868–1878. https://doi.org/10.2307/1940038
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C: N: P stoichiometry in forests worldwide: implications of terrestrial Redfield-type ratios. Ecology 85:2390–2401. https://doi.org/10.1890/03-0351
Nambiar EKS, Fife DN (1987) Growth and nutrient retranslocation in needles of radiata pine in relation to nitrogen supply. Ann Bot 60:147–156. https://doi.org/10.1093/oxfordjournals.aob.a087431
Reed SC, Townsend AR, Davidson EA, Cleveland CC (2012) Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol 196:173–180. https://doi.org/10.1111/j.1469-8137.2012.04249.x
Reich PB (2005) Global biogeography of plant chemistry: filling in the blanks. New Phytol 168:263–266. https://doi.org/10.1111/j.1469-8137.2005.01562.x
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci U S A 101:11001–11006. https://doi.org/10.1073/pnas.0403588101
Rentería LY, Jaramillo VJ (2011) Rainfall drives leaf traits and leaf nutrient resorption in a tropical dry forest in Mexico. Oecologia 165:201–211. https://doi.org/10.1007/s00442-010-1704-3
Rentería LY, Jaramillo VJ, Martínez-Yrízar A, Pérez-Jiménez A (2005) Nitrogen and phosphorus resorption in trees of a Mexican tropical dry forest. Trees 19:431–441. https://doi.org/10.1007/s00468-004-0402-3
Richardson SJ, Peltzer DA, Allen RB, McGlone MS (2005) Resorption proficiency along a chronosequence: responses among communities and within species. Ecology 86:20–25. https://doi.org/10.1890/04-0524
Sabaté S, Sala A, Gracia CA (1995) Nutrient content in Quercus ilex canopies: seasonal and spatial variation within a catchment. Plant Soil 168:297–304. https://doi.org/10.1007/bf00029341
Savolainen O, Pyhäjärvi T, Knürr T (2007) Gene flow and local adaptation in trees. Annu Rev Ecol Evol Syst 38:595–619. https://doi.org/10.1146/annurev.ecolsys.38.091206.095646
Scalon MC, Wright IJ, Franco AC (2017) To recycle or steal? Nutrient resorption in Australian and Brazilian mistletoes from three low-phosphorus sites. Oikos 126:32–39. https://doi.org/10.1111/oik.03455
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195:260–262. https://doi.org/10.1126/science.195.4275.260
See CR, Yanai RD, Fisk MC, Vadeboncoeur MA, Quintero BA, Fahey TJ (2015) Soil nitrogen affects phosphorus recycling: foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 96:2488–2498. https://doi.org/10.1890/15-0188.1
Shi C, Han R, Wu P, Fang M, Lai H, Shentu L (2011) Geochemistry and provenance of source rock for matoushan formation rocks (late cretaceous) in the Mouding area, Central Yunnan. Acta Sedimentol Sin 29:303–311. https://doi.org/10.14027/j.cnki.cjxb.2011.02.013
Sun X, Kang H, Chen HYH, Björn B, Samuel BF, Liu C (2016) Biogeographic patterns of nutrient resorption from Quercus variabilis Blume leaves across China. Plant Biol 18:505–513. https://doi.org/10.1111/plb.12420
Sun X, Kang H, Kattge J, Gao Y, Liu C (2015) Biogeographic patterns of multi-element stoichiometry of Quercus variabilis leaves across China. Can J For Res 45:1827–1834. https://doi.org/10.1139/cjfr-2015-0110
Tao Y (2005) A proximate mathematical model of the content of main chemical constituents in an industrial phosphorus ore. Yunnan Geol 2:003. https://doi.org/10.3969/j.issn.1004-1885.2005.02.003
Turner BL, Laliberté E (2015) Soil development and nutrient availability along a 2 million-year coastal dune chronosequence under species-rich Mediterranean shrubland in southwestern Australia. Ecosystems 18:287–309. https://doi.org/10.1007/s10021-014-9830-0
Vadeboncoeur MA (2010) Meta-analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Can J For Res 40:1766–1780. https://doi.org/10.1139/X10-127
Van Heerwaarden LM, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669. https://doi.org/10.1034/j.1600-0706.2003.12351.x
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. https://doi.org/10.1890/11-0416.1
Vitousek PM (1998) Foliar and litter nutrients, nutrient resorption, and decomposition in Hawaiian Metrosideros polymorpha. Ecosystems 1:401–407. https://doi.org/10.1007/s100219900033
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19. https://doi.org/10.1046/j.1365-2435.2003.00694.x
Wu Z, Zhu Y, Jiang H (1987) The vegetation of Yunnan. Science, Beijing
Xiao G, Li T, Zhang X, Yu H, Huang H, Gupta DK (2009) Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area. J Hazard Mater 171:542–550. https://doi.org/10.1016/j.jhazmat.2009.06.034
Yan K, Fu D, He F, Duan C (2011) Leaf nutrient stoichiometry of plants in the phosphorus-enriched soils of the Lake Dianchi watershed, southwestern China. Chin J Plant Ecol 35:353–361. https://doi.org/10.3724/SP.J.1258.2011.00353
Yuan Z, Chen HYH (2009a) Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob Ecol Biogeogr 18:11–18. https://doi.org/10.1111/j.1466-8238.2008.00425.x
Yuan Z, Chen HYH (2009b) Global trends in senesced-leaf nitrogen and phosphorus. Glob Ecol Biogeogr 18:532–542. https://doi.org/10.1111/j.1466-8238.2009.00474.x
Yuan Z, Chen HYH (2012) A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc R Soc Lond B Biol Sci 279:3796–3802. https://doi.org/10.1098/rspb.2012.0955
Yuan Z, Chen HYH (2015) Negative effects of fertilization on plant nutrient resorption. Ecology 96:373–380. https://doi.org/10.1890/14-0140.1
Yuan Z, Li L, Han X, Chen S, Wang Z, Chen Q, Bai W (2006) Nitrogen response efficiency increased monotonically with decreasing soil resource availability: a case study from a semiarid grassland in northern China. Oecologia 148:564–572. https://doi.org/10.1007/s00442-006-0409-0
Zhang D (2017) rsq. partial: Partial R-Squared for Generalized Linear Models. https://CRAN.R-project.org/package=rsq
Zhou X, Sun X, Du B, Yin S, Liu C (2015) Multielement stoichiometry in Quercus variabilis under natural phosphorus variation in subtropical China. Sci Rep 5:7839. https://doi.org/10.1038/srep07839
Acknowledgements
We thank the Instrumental Analysis Center of Shanghai Jiao Tong University for the support on chemical analysis.
Funding
This study was funded by the National Key R&D Program of China (2016YFC0502501) and the National Natural Science Foundation of China (No. 31670626, No. 31270640, and No. 31070532).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Andrew Merchant
Contribution of the co-authors
C. Liu conceived and designed the experiments and wrote the manuscript. J. Wen, B. Du, and N. Sun performed the experiments. B. Berg analyzed the data. H. Ji performed the experiments, analyzed the data, and wrote the manuscript.
Electronic supplementary material
ESM 1
(PDF 189 kb)
Rights and permissions
About this article
Cite this article
Ji, H., Wen, J., Du, B. et al. Comparison of the nutrient resorption stoichiometry of Quercus variabilis Blume growing in two sites contrasting in soil phosphorus content. Annals of Forest Science 75, 59 (2018). https://doi.org/10.1007/s13595-018-0727-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-018-0727-5