Abstract
Introduction
Silicones (e.g., dimethicone) are effective and safe alternatives to insecticides for the treatment of head lice. However, silicones are lipophilic substances and do not only leave the hair greasy but they are also difficult to wash out. We have evaluated the efficacy and safety of a potential solution to this problem: an aqueous dispersion of a novel silylated polyol that has the same mode of action as dimethicone (suffocation) without its negative impact on hair characteristics.
Methods
This was a randomized, controlled, investigator-blinded, bicentric study that was conducted at two locations in the state of Florida (USA) to compare the test product (medical device) to a pyrethrum-based pediculicide that is a first-line, prescription-free treatment against head lice in the USA. The subjects (n = 70) were randomly divided into two groups of 35 persons (test product group and reference product group), with each participant receiving two applications (day 0 and 7) of the product to be tested, according to the instructions for use. Efficacy and safety was evaluated at distinct time points. The primary objective was to establish a cure rate for the test product that was better than 70% at study end (day 10). Esthetic effects of the test product versus dimethicone were evaluated in a blinded, cross-over consumer study (n = 100).
Results
At study end, the cure rate (corrected for re-infestation) of 88.2% with the test product significantly surpassed the pre-defined target of 70%, and thus the superiority of the test product versus the reference product was confirmed. The number of subjects cured (free of head lice) after the first treatment was remarkably higher with the test product than with the reference product (57.1 vs. 2.9%, respectively). Both products were safe and well tolerated and both showed beneficial esthetical effects. The consumer test demonstrated that the test product had better washing-out properties than dimethicone, as reflected by a significantly lower average rinsing time and number of washings required to restore the visual aspect of the hair, especially in terms of greasiness.
Conclusion
Aqueous dispersions of silylated polyols are a promising new class of pediculicides that combine high cure rates with optimal user convenience (short treatment period, easy wash-out with positive effect on hair quality).
Trial Registration
ClinicalTrials.gov identifier, NCT03617926.
Funding
Oystershell Laboratories.
Similar content being viewed by others
Introduction
Head lice (Pediculus humanus capitis) are hematophagous ectoparasites which live on the human scalp. They are very contagious and typically spread via close contact with infested individuals. Head lice are common and widely spread, with highly diverging prevalence values [1, 2].
Because of increasing resistance and safety issues [3, 4], the use of insecticides (e.g., pyrethroids) as first-line agents against head lice has been largely surpassed by physically acting, substance-based medical devices that typically contain silicones and/or mineral oils. The physical mode of action of these devices is widely accepted and relies on suffocation of the ectoparasites by blocking their respiratory channel, hereby impairing water and gas exchange. Mineral oils are also able to disrupt the epicuticular wax layer of head lice [5,6,7,8].
Such agents have a lipophilic character, and especially high-molecular-weight silicones can accumulate on the hair, making them difficult to wash out and therefore turning the hair greasy [9]. These negative properties have led researchers to search for alternative, physically active compounds that combine efficacy and optimal user convenience in terms of treatment period and with the lack of negative effects on hair quality. Different screening efforts have resulted in the identification of a group of synthesized, silylated polyols, which show the most interesting properties with respect to adulticidal and ovicidal activity (100% mortality). Furthermore, in contrast to the high-molecular-weight silicones, short silylated polyols have a very low viscosity, allowing easy dispersion in viscous aqueous formulations. From an esthetical point of view, these molecules are promising as they do not leave residue on the hair and do not have a negative impact on hair quality in general.
The clinical trial described here was designed and implemented to compare the efficacy, safety, and cosmetic effects of an aqueous dispersion of a silylated polyol (Prosil) versus a first-line, prescription-free treatment in the USA: RID shampoo (pyrethrum-based). We also performed a blinded, cross-over consumer study to further evaluate the esthetic effects of the test product versus a silicone-based product (4% dimethicone).
Methods
Clinical Study Set-Up
This randomized, controlled, investigator-blinded, comparative study was approved by the Schulman IRB Ethics Committee (Cincinnati, OH, USA) on 16 January 2018. The trial was conducted according to the international guideline for clinical trials with pediculicides [10], in accordance with Good Clinical Practice and with the principles of the Declaration of Helsinki 2013. This study was registered at ClinicalTrials.gov (NCT03617926).
The entire clinical study took place at two clinical trial sites in Florida (USA) that are specialized in treating people infested with head lice (site 1: Lice Source Services, Plantation, FL; site 2: Lice Cleanique, Miami, FL). Recruitment was performed by trained personnel and continued from 7 March 2018 (first visit of first patient) to 30th June 2018 (last visit of last patient). Patients were recruited among infested subjects who visited one of either clinic for a head lice treatment.
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria were identical to those used in the study of Wolf et al. [11]. Briefly, patients (> 2 years of age; male/female) were included after head lice infestation was confirmed. Full scalp and hair diagnoses were performed by experienced clinical staff using a plastic head lice comb. First, the hair was detangled into three sections, and every section was combed six times, beginning at the scalp and ending at the hair tips. Head lice caught by the comb were left in the hair to avoid bias. The severity of the head lice infestation was classified as follows: not relevant (< 5 lice and/or nymphs), mild (5–9 lice and/or nymphs), moderate (10–24 lice and/or nymphs), and severe (≥ 25 lice and/or nymphs).
Only patients with a mild, moderate, or severe infestation, with at least five apparently live nits (based on location and visual appearance), and a good general condition were included in the study. Exclusion criteria were: sensitivity towards ingredients; use of a pediculicide 1 month prior to and during the study; use of topical drugs or medication which may interfere with study outcome; scalp disorders; pregnancy or lactation; participation in another clinical trial (also taking into account a period of 4 weeks before study start); being affiliated to the sponsor or investigator site personnel; and non-compliance with the protocol.
Informed Consent, Randomization, and Baseline Data
During recruitment, the clinical staff provided detailed information on study procedures and objectives. Following written consent by the participant (or his/her legal representative), each subject was randomly allocated into one of two groups. Baseline demographic and clinical data were collected. Prior to the study, a randomization list was built by an external statistician using SAS software (version 9.4; SAS Institute, Cary, NC, USA). For this purpose, block randomization with a variable block size (2–8) was used. All patients confirmed their availability for the 10-day duration of the study.
Blinding
Because of differences in product characteristics (color, odor, composition…), a double-blinded study was not possible. Therefore, treatments were provided by unblinded study staff, whereas consequent assessments were performed by blinded study staff (not involved in the treatments) to avoid any product bias.
Study Products, Dosage, and Administration
Both products were applied according to the respective instructions for use. The test product (medical device) and corresponding lice comb were supplied by Oystershell (Merelbeke, Belgium). The lotion is provided as a dual chamber packaging system. Briefly, the cap contains the concentrated silylated polyol (Prosil), which is dissolved in a lipophilic vehicle. The 100-ml plastic bottle contains the water diluent (supplemented with preservatives, perfume, and a viscosity modifier). Prior to use, the cap is activated by pushing, hereby releasing the Prosil into the water phase. Next, the product is shaken thoroughly and immediately applied onto dry hair until the hair is fully saturated. After 15 (+ 2) min, the hair is rinsed with warm water and a commercial mild shampoo (Johnson’s baby shampoo; Johnson & Johnson, New Brunswick, NJ, USA).
The reference product, namely RID shampoo, is commercially available in the USA. It contains pyrethrum extract (equivalent to 0.33% pyrethrins) and piperonyl butoxide (4%), with the latter serving as activity booster. Pyrethrum is extracted from flower heads of Chrysanthemum cinerariifolium and contains pyrethrins, cinerins, and jasmolins. The extract has neurotoxic effects and kills insects, including head lice, by acting on their central nerve system (disruption of muscle neurotransmission) [3, 12]. The clinical trial centers purchased RID shampoo from stores as 118-ml glass bottles, supplemented with a lice comb.
Depending on the length of the hair, the following volumes were applied to the hair (according to the instructions for use): 60 ml (short hair), 90 ml (shoulder length-hair), and complete bottle (mid-back hair), respectively. The product was applied evenly in dry hair over its full length, with special attention paid to the hair and scalp. After 10 (+ 2) min, warm water was applied, and the product was rinsed out thoroughly.
Patients received two treatments, a first application at visit 1 (day 0) and a second at visit 3 (day 7), according to the instructions for use of both products.
Efficacy Evaluation
Infestation degree in both treatment groups was diagnosed using a plastic head lice comb. Assessments were made at the following time points: day 0 (visit 1), 24 h post treatment (visit 2), day 7 post treatment (prior to second treatment; visit 3), and day 10 post treatment(visit 4).
The primary objective of this study was to demonstrate that the cure rate of the test product surpassed 70% at day 10. The cure rate is the proportion of patients without any living lice, corrected for re-infestation and was defined as (1) no adult lice or third-stage nymphs present following the first treatment, and (2) no more than two adult lice or third-stage nymphs found by combing on day 10 [13].
The secondary efficacy objectives were to show (1) that the cure rate at the end of day 10 (corrected for re-infestation) for the reference product surpassed 70%, and (2) that the test product had a cure rate superior to that of the reference product. In the case that superiority could not be shown, the secondary efficacy objectives were to demonstrate that the test product was non-inferior to the reference product, using a non-inferiority margin of 7.5%. More detailed information is provided in the Statistical Analyses section.
Sample Size Calculation
Sample size calculation relied on previous unpublished and published clinical data [11]. A pre-defined limit of 70% was chosen, based on the highest cure rate reported to date, from a clinical trial with a pyrethroid-based pediculicide [14]. This limit was assumed to be the minimal acceptable cure rate. A sample size of 30 was needed for a one-sample Chi-square test, comparing a cure rate of 95% with a fixed limit of 70% (two-sided test; alpha level of 0.05; power 80%). For the control group, an identical sample size was used.
Assuming a 10% drop-out and a 5% re-infestation rate, the sample size was multiplied by 1.1 × 1.05, resulting in 35 cases per group (n = 70 in total).
Safety Evaluation
Assessment of safety, tolerability, and acceptance of both products were some of the secondary objectives. Such assessments were performed before and after the first (day 0) and second treatment (day 7), respectively. A final examination was done at study end (day 10) to assess local tolerability, as reflected by the presence of burning, pruritus, and paresthesia. Study staff evaluated skin condition (secondary infection, skin redness, excoriation) and ocular irritation on days 0, 1, 7, and 10, respectively. Global tolerability was evaluated by the clinical staff at study end.
Esthetic Questionnaire
Following application of the first and second treatment, an aesthetic questionnaire was completed by the subjects on days 0 and 7, respectively. The questions related to dryness, shininess, greasiness, and volume of the hair; feeling of the hair and scalp; and the conditioning effect of the treatment. The following scores were assigned: (a) strongly agree, (b) agree, (c) disagree, and (d) strongly disagree.
Consumer Study
The blinded, cross-over study was designed and implemented by VG Sensory (Deinze, Belgium), a firm which is specialized in conducting consumer tests and evaluating sensory properties of cosmetics, food products, among others. The study started on 9 August 2018 and finished on 25 September 2018. Subjects (n = 100) were recruited from a database of people with home experience in the use of head lice products. In this study, the test product was compared to a commercially available 4% dimethicone lotion. People were instructed to use the product according to the respective leaflet. A commercially available mild shampoo was used to wash out the product (same for both treatments). At study start, 50 subjects started with one test product and the remaining 50 with the other. Following treatment, a “wash-out” period of 14 days had to be respected before the participants started on the other treatment. Different parameters were recorded in a diary: time required to wash out the product; the number of washings required to restore hair characteristics; and the volume of shampoo used. All subjects completed a questionnaire to score the esthetic aspects of the hair, including greasiness, shininess, feel, and of the scalp, among others.
In vitro Adulticidal Tests and Microscopical Analyses
Living head lice were collected from a school in the direct environment of Oystershell Laboratories and immediately used in an in vitro adulticidal test. Briefly, all stages of viable head lice (n = 10) were transferred to a self-made “arena”, which was an enclosed circle of nylon gauze. Next, head lice were treated with 2 ml of the test product, namely, 4% dimethicone lotion (positive control), or with 2 ml tepid water (negative control) for 15 min. Following incubation, lice were rinsed with a 5% dilution of a commercial shampoo and water. Survival was assessed at distinct time points for a period of up to 3 h. Treated lice (all products) were then transferred to the LIMID laboratory (University of Antwerp, Belgium) for microscopical analysis to evaluate penetration of test product in the respiratory tract. This method was based on experiments performed by Richling [15]. Imaging of the lice was performed with a Zeiss Stemi SV11 stereomicroscope (Carl Zeis NV, Zaventem, Belgium), equipped with an Olympus DP70 CCD Camera (Olympus, Antwerp, Belgium).
Statistical Analyses
Clinical trial data were analyzed by an independent statistician according to the International Conference on Harmonization (ICH) E9 Note for Guidance on Statistical Principles for Clinical Trials [16].
Efficacy analyses were performed on the intention-to-treat (ITT) population. The latter included all subjects who were recruited and randomized in the study, with baseline values of the primary endpoint, and at least one follow-up visit for the efficacy parameters. A 5% level of significance level was applied for analyses.
The cure rate (p) corresponds to the proportion of patients who were cured at day 10 among all patients that received any treatment at day 0. The aim of this study was to establish superiority for the cure rate of the test product (pT) versus a pre-defined limit of 70% (see also section Sample size). The following null hypothesis was tested: H0, prim: pT − pR = 0 and Ha: pT − pR > 15%, where pR is the cure rate of the reference product. The Chi-square test of independence was used to test the null hypothesis. If superiority could not be proven, non-inferiority would be evaluated. Evaluation of non-inferiority is allowed if a non-inferiority margin has been predefined, and no correction were made in terms of multiplicity [17]. As non-inferiority margin (δ), a 7.5% worse cure rate was seen as being clinically not relevant. In the present clinical trial, this analysis was not relevant as results clearly confirmed superiority of the test product.
The Chi-square test for independence was used to compare the cure rate after correction for re-infestation on day 10.
All descriptive and statistical analyses were performed in R version 3.3.4. (R development core team 2017) [18]. A p value of < 0.05 was considered to be statistical significant. No imputation of missing data was performed.
For the consumer study, continuous data were summarized by their mean, standard deviation (SD), minimum, median, and maximum. Categorical data were summarized by frequencies and percentages. Fizz Sensory Analysis software (Biosystemes Analysis, Couternon, France) was used to perform analysis of variance tests. For time measurement data, a Mann–Whitney test was used. All statistical tests were performed at the 5% level of significance.
Results
Baseline Data Clinical Trial
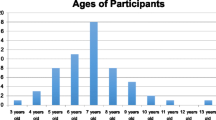
Study data were collected between 7 March 2018 and 30 June 2018. In total, 70 subjects were randomized into two treatment groups, namely, the test product group (32 females, 3 males) and the reference product group (33 females, 2 males). The average age of the participants was 14.2 ± 11.7 (± SD) years, with age ranging from 2 to 50 years. All patients completed the study according to the protocol and were included into the ITT population. A CONSORT flow chart is shown in Fig. 1. A summary of demographic and clinical characteristics is presented in Table 1.
Forty patients (n = 20 in both treatment groups) were classified as having a mild infestation of head lice, and 21 patients were assessed as having a moderate infestation (test product group, n = 8; reference group, n = 13). Compared to the reference product group, more subjects in the test product group suffered from a severe infestation (n = 7 vs. n = 2, respectively).
Before treatment (visit 0), the average number of adult live lice per subject was 6.3 ± 7.2 (range 0–40). The average number of adult lice and the distribution of nymphs per stage differed slightly between treatment arms.
The number of subjects with short hair was comparable in both groups (test product group n = 3; reference product group n = 4). With respect to hair length and treatment for head lice infestation, there were more subjects in the reference group (n = 16) with medium long hair (shoulder length) treated for head lice than in the test product group (n = 12), while in contrast, there were more subjects in the test product group (n = 20) with long hair (mid-back) treated for head lice infestation than in the reference group (n = 15). Regarding hair curliness and thickness, no important differences were observed between both treatment groups. The data are summarized in Table 1.
Efficacy Results
At study end (day 10), only five of 35 subjects were infested in the test product group (cure rate 85.7%). One subject was assessed to be a re-infestation, yielding a cure rate, corrected for re-infestation, of 88.2% (30/34; 95% confidence interval [CI] 72.5–96.7%). At study end, 22 patients in the reference product group were not lice-free (cure rate 37.1%). Three subjects were classified as a re-infestation, yielding a cure rate, corrected for re-infestation, of 40.6% (13/32; 95% CI 23.7–59.4%). These data demonstrate that the primary objective (cure rate > 70%) was only realized for the test product (p = 0.023). With a difference between cure rates of 47.6% (95% CI 27.4–67.8%; χ2: 16.46, p < 0.001), superiority of the test product versus reference product was statistically established.
For all evaluation visits, cure rates were remarkably higher for the test product than for the reference product. Twenty-four hours following initial treatment, 85.7% (30/35) and 60% (21/35) of the subjects were lice-free in the test group and reference group, respectively. Prior to the second treatment, there was a significant advantage of 40% for the test product: 51.4% of the subjects in the test product group were lice-free versus 11.4% in the reference product group.
After the first treatment, the number of subjects who remained lice-free until study end was 57.1% (20/35) in the test product group and 2.9% (1/35) in the reference product group. The results are shown in Table 2.
Satisfaction with Esthetical Effect
In the test product group, 91–100% of the subjects responded positively to all questions, whereas no subjects strongly disagreed. Briefly, after the first treatment, 6% of the subjects in this group disagreed with the statement “Hair not too dry”; after the second treatment, only 3% disagreed. Identical results were reported regarding the conditioning effect of the product. Regarding the statements on shininess (Shininess is good) and volume of the hair (Volume is good), 3% of the subjects in the test product group disagreed after the first treatment, whereas none of the subjects in this group disagreed after the second treatment. Finally, after the first treatment, 9% of the subjects disagreed with the statement “Greasiness is normal”; however, at the end of the study, only 3% disagreed.
In the reference product group, 94–100% of the subjects responded positively to all statements, with the exception for that on dryness of the hair. Indeed, 14 and 9% of the subjects in this group disagreed with the statement that the hair does not feel too dry after the first and second treatment, respectively. Furthermore, 3% of the subjects strongly disagreed and 3% disagreed with the statement that the hair feels good after the first treatment; after the second treatment, none of the subjects disagreed.
With respect to the statements on shininess and volume of the hair, and the conditioning effect, the outcomes were comparable in both groups for both treatments. With regards to the statement on greasiness of the hair, 3% or none of the subjects in the reference product group disagreed after the first and second treatment, respectively. The results of this questionnaire are presented in detail in Table 3.
Safety Evaluation
Local Tolerability
Local tolerability was assessed during all visits, with the following parameters being assessed: burning, paraesthesia, and pruritus. In the test product group, paraesthesia and burning were not reported. Prior to treatment, pruritus was reported by 77% of the subjects (mild 43%, moderate 23%, severe 11%). At each follow-up visit, the number of subjects reporting pruritus gradually decreased. At study end, only 20% of the subjects reported mild pruritus. Moderate to severe pruritus was not observed. In the reference group, paraesthesia was not reported. Only one subject in this group complained about mild burning before the first treatment, but this did not reoccur. Prior to treatment, pruritus was reported by 69% of the subjects (mild 37%, moderate 23%, severe 9%). At each follow-up visit, the number of subjects reporting pruritus gradually decreased. At study end, 14% reported mild pruritus (no moderate or severe cases). Moderate to severe pruritus was not observed. These results are shown in more detail in Table 4.
Global Tolerability
At study end, global tolerability was rated as “good” or “very good”. The number of subjects in the test product group with the rating “very good” was remarkably higher than that in the reference product group (65.7 vs. 48.6%, respectively).
Skin Irritations
Skin erythema was not reported in the test product group. In the reference group, one subject reported mild erythema (24 h post treatment), but this did not reoccur at days 7 and 10, respectively. Scalp excoriation and secondary infections were not reported in both groups.
Eye Irritations
For both investigational products, eye tolerability was excellent. None of the subjects suffered from eye redness and irritation over all visits.
Adverse Events and Serious Adverse Events
In both treatment groups, no (serious) adverse events were reported.
Consumer Study Outcome
The test product scored significantly better in view of wash-out time. Briefly, an average wash-out time of 17.5 (95% CI 15.1–20.0) min and 11.0 (95% CI 9.3–12.8) min was calculated for the dimethicone reference product and the test product, respectively. This difference was significantly different (p < 0.0001). The shorter wash-out time was also confirmed by the responses to the open comments: 62% of the test subjects indicated spontaneously that the treatment with the dimethicone product was time-consuming.
In case the visual aspect of the hair was not good, consumers were allowed to perform additional washings during the days following treatment. Briefly, a higher number of subjects performed extra washings in the dimethicone reference group (41 vs. 30% with test product), with an average wash-out time of 12.5 and 10.6 min for the dimethicone reference product and test product, respectively. This difference was not significant. Combining the time for initial washing and the extra washing time, an average of 22.6 min was calculated for the dimethicone reference product, whereas treatment time with test product was significantly (p > 0.001) lower (14.2 min). Of note, in the test product group, 22% of the subjects only required one washing, whereas 71% of the subjects positively scored the visual aspect of the hair after two to three washings. In contrast, significantly more washings (up to 7 or more) were required for subjects treated with the dimeticone product. Despite this higher number of washings, the hair was still assessed as significantly greasier by the subjects in this group (39% of the subjects indicated that the hair was too greasy). The feel of the scalp and the feel of the hair scored better with test product but did not significantly differ from the results, obtained with the reference product.
Discussion
In recent years, insecticide-based products for the treatment of pediculosis capitis have been gradually replaced by alternative products with a physical mode of action, with the primary drivers of this change being growing resistance of head lice to the agents and safety concerns [3, 4]. Many of these newer products contain silicones (e.g., high-molecular-weight dimethicone) and/or mineral oils. Their physical mode of action relies on suffocation and dehydration of the head lice [5, 7, 8]. Although these products have been proven to be safe and to have clinical efficacy [6, 11, 19, 20], they may have a major disadvantage from a cosmetic point of view, silicones in particular. Indeed, because of their lipophilicity, these products may leave a residue on the hair and make it oily and greasy [9].
Extensive research has revealed that silylated polyols may be interesting alternatives to silicones. Independent in vitro experiments have shown that the test product in this clinical trial, an aqueous dispersion of one of these molecules (Prosil), yields 100% mortality for both lice and nits. Prosil is an interesting molecule because it elicits its effects in an identical manner as dimethicone. Indeed, our microscopic analyses revealed that, following treatment, the respiratory channels of the head lice were completely filled with product in a manner comparable to dimethicone exposure. Furthermore, treated head lice appeared to be highly desiccated (see Fig. 2). Interestingly, in contrast to silicones, no build up effect on hair strands treated in vitro with the aqueous Prosil dispersion was observed.
From a chemical point of view, Prosil and other silylated polyols differ from silicones in general. Indeed, the latter consists of a siloxane (Si–O–Si) bone structure, whereas the active ingredient only contains organosilicon (Si–O–C) bonds. Both types of molecules are insoluble in polar solvents such as water. However, because of their very low viscosity, silylated polyols can be easily dispersed and stabilized in an aqueous vehicle with medium viscosity. This is not the case for high-molecular-weight silicones, which are very viscous. One could opt for emulsification (oil in water emulsion) to “dissolve” the silylated polyol into the water phase. However, in vitro experimental data have shown that adulticidal activity of the agent was significantly reduced (60% mortality) after emulsification. The most plausible hypothesis for this loss of activity after emulsification is that a permanent oil-in-water emulsion rinses off too easily from the hair and, consequently, the product does not have sufficient time to affect the head lice. This possibility is consistent with the notion that penetration of silicone or oil compounds into the spiracles and respiratory tract is essential to kill head lice and nits. It has recently been shown that water is not able to penetrate the respiratory system of the head lice [21], a finding which further supports our hypothesis.
Silylated polyalcohols have been observed to self-emulsify in polar solvents over time. To avoid such self-emulsification, a dual chamber packaging was developed. Briefly, the silylated polyol (Prosil) is dissolved in a water-free, lipophilic vehicle, which is stored in the cap. Following activation of the cap, this Prosil phase is released into the aqueous vehicle (containing a viscosity modifier, a perfume, and preservatives). Following shaking, the product can be directly applied to the hair. Interestingly, the formulation remains stable for several weeks without any loss of in vitro activity and without phase separation (probably because of the relatively high viscosity of the product).
The present clinical trial demonstrated that treatment with the test product resulted in a high cure rate of 88.2% at day 10 (corrected for re-infestation), amply exceeding the pre-defined limit of 70%. The latter value lies closely to the highest cure rate (67.7%) reported in a recent clinical trial with a pyrethrum-based pediculicide [14]. It is important to stress that, at present, this is a rather exceptional outcome. Much lower cure rates have been observed with comparable treatments [3, 22,23,24,25,26,27], and such lower cure rates are supported by the results obtained with the reference product in the present study. Indeed, over all visits, cure rates were remarkably higher for the test product than for the control at each visit, with a final cure rate of 40.6% (corrected for re-infestation). Furthermore, one single application of the test product was sufficient to keep 57.1% of all patients lice-free until study end, in contrast to 2.9% in the reference product group.
The most logic explanation for the observed results is the growing resistance of head lice towards insecticides. Indeed, over the last decades, cure rates significantly reduced, with levels dropping to 25% [28]. Knockdown resistance (kdr)-type mutations, resulting in nerve insensitivity to pyrethrins and derivatives, are responsible for this phenomenon. A recent study in the USA showed a high percentage of lice (up to 100%) with kdr-type mutations in all U.S. states [29]. The present study was performed in two centers in Florida, which were close to each other. For this reason, a similar resistance profile can be expected in both sites.
In general, insecticide resistance is a worldwide problem and is related to misuse and overexposure. One recent study, performed in Germany, reported an exceptionally high cure rate of 94% following two treatments with a pyrethrum-based lotion. The authors stated that this may be related to the fact that the insecticide-based pediculicides require a prescription and, therefore, people are less exposed [11]. Despite growing resistance, pyrethrin- and pyrethroid-based products still belong to the first-line, prescription-free treatments available in the USA, although substance-based pediculicides have shown to be superior in view of performance and safety [6, 11, 19, 20]. As the study was performed in Florida, RID shampoo was used as reference in the present study.
In addition to clinical performance, the esthetic effects of the test product were evaluated during the trial. Subjects highly appreciated the cosmetic effects of the test product following both applications at day 0 and day 7, respectively. Indeed, more than 90% of the test subjects were (very) satisfied with the condition of their hair (shininess, volume, feeling of hair and scalp, dryness, and greasiness). This was further confirmed by the results of the blinded, cross-over consumer study, which showed that a significantly lower rinsing time and number of washings were required to restore the hair in its original form.
With respect to safety, the aqueous lotion consists of ingredients which are approved for use in pharmaceutical and cosmetic products. The active ingredient has a comparable chemical structure to other silylated products which are applied in a variety of cosmetic products. The clinical results showed that the product was well-tolerated, as reflected by the absence of any adverse events (including skin and eye irritation). Similar observations were made for the comparator product.
The limitations of this study include the moderated number of subjects. However, sample size was calculated using the data of earlier clinical trials with silicone- or mineral oil-based pediculicides. Power analysis confirmed that this scale of significant differences between treatments is detectable in a study population of n = 50–100. Instead of a pyrethroid-based product, a silicone-based pediculicide might have served as reference, especially in view of the increasing resistance. Also, no subgroup analyses were performed to evaluate the impact of infestation degree, hair characteristics, and other parameters. However, no remarkable differences were found in baseline parameters between both treatment groups. Furthermore, subgroup analyses were not foreseen in the original statistical plan. Subgroup analyses may reveal some trends but because of the relatively low number of subjects, the power of the tests would be insufficient and, therefore, any conclusions may not be correct, statistically seen. Finally, dry combing was used to diagnose a head lice infestation in both treatment groups. This method may be less sensitive compared to wet combing. However, the latter requires the use of a conditioner, which in turn may have an impact on lice and thus on study outcome. For this reason, wet combing was not used as a diagnostic tool.
Conclusions
In conclusion, the results of the present study demonstrated that treatment with an aqueous dispersion of a silylated polyol resulted in high cure rates compared to a pyrethrum-based, prescription-free pediculicide shampoo. In addition to the high safety profile of the test product, its esthetic effects following application were assessed to be excellent, in contrast to the assessment of silicone-based head lice treatments, showing comparable cure rates but having negative effects on hair quality due to wash-out difficulty and residue build-up.
References
Falagas ME, Matthaiou DK, Rafailidis PI, Panos G, Pappas G. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493–4.
Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105–10.
Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect. 2012;18:338–44.
Saillenfait AM, Ndiaye D, Sabaté JP. Pyrethroids: exposure and health effects—an update. Int J Hyg Environ Health. 2015;218:281–92.
Burgess IF. The mode of action of dimethicone 4% lotion against head lice, Pecidulus capitis. BMC Pharmacol. 2009;9:1–8.
Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin crème rinse for treatment of head louse infestation: a randomised, controlled trial. BMC Dermatol. 2013;13:5.
Agnello AM. Petroleum-derived spray oils: chemistry, history, refining and formulation. In: Beattie GAC, Watson DM, Stevens ML, Rae DJ, Spooner-Hart- RN, editors. Spray oils—beyond 2000. Sidney: University of Western Sydney and Horticulture Australia Ltd.; 2002. p. 2–18.
Taverner P. Drowning or just waving? A perspective on the ways petroleum-derived oils kill arthropod pests of plants. Spray oils—beyond 2000. Sidney: University of Western Sydney and Horticulture Australia Ltd.; 2002. p. 78–87.
Dias MFRG. Hair cosmetics: an overview. Int J Trichol. 2015;7:2–15.
Barker SC, Burgess IF, Meinking TL, Mumcuoglu KY. International Guidelines for clinical trials with pediculicides. Int J Dermatol. 2012;51:853–8.
Wolf L, Eertmans F, Wolf D, Rossel B, Adriaens E. Efficacy and safety of a mineral oil-based head lice shampoo: a randomized, controlled, investigator-blinded, comparative study. PLoS One. 2016;11:e0156853.
Taplin D, Meinking TL. Pyrethrins and pyrethroids in dermatology. Review and editorial. Arch Derm. 1990;126:213–31.
Burgess IF. 1, 2-Octanediol, a novel surfactant, for treating head louse infestation: identification of activity, formulation, and randomised, controlled trials. PLoS One. 2012;7(4):e35419.
Heukelbach J, Pilger D, Oliveira FA, Khakban A, Ariza L, Feldmeier H. A highly efficacious pediculicide based on dimeticone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
Richling I, Böckeler W. Lethal effects of treatment with a special dimeticone formula on head lice and house crickets (Orthoptera, Ensifera: acheta domestica and Anoplura, phthiraptera: Pediculus humanus). Insights into physical mechanisms. Arzneimittelforschung. 2008;58:248–54.
International Conference on Harmonization (ICH). Statistical principles for clinical trials. Federal Register. 1998;63:49583.
International Conference on Harmonization (ICH). Points to consider on switching between superiority and non-inferiority. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003658.pdf.
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Burgess IF, Brown CM, Lee PN. Treatment of head louse infestation with 4% dimethicone lotion: randomised controlled equivalence trial. BMJ. 2005;330:1423.
Kurt O, Balcioğlu IC, Burgess IF, Limoncu ME, Girginkardeşler N, Tabak T, et al. Treatment of head lice with dimeticone 4% lotion: comparison of two formulations in a randomised controlled trial in rural Turkey. BMC Public Health. 2009;9:441.
Candy K, Brun S, Nicolas P, Durand R, Charrel RN, Izri A. Do drowning and anoxia kill head lice? Parasite. 2018;25:8.
Burgess IF, Lee PN, Matlock G. Randomised, controlled, assessor blind comparing 4% dimethicone lotion with 0.5% malathion liquid for head louse infestation. PLoS One. 2007;2:e1127.
Barker SC, Altman PM. A randomised, assessor blind, parallel group comparative efficacy trial of three products for the treatment of head lice in children—melaleuca oil and lavender oil, pyrethrins and piperonyl butoxide, and a “suffocation” product. BMC Dermatol. 2010;10:6.
Gallardo A, Mougabure-Cueto G, Vassena C, Picollo MI, Toloza AC. Comparative efficacy of new commercial pediculicides against adults and eggs of Pediculus humanus capitis (head lice). Parasitol Res. 2012;110:1601–6.
Burgess IF, Kay K, Burgess NA, Brunton ER. Soya oil-based shampoo superior to 0.5% permethrin lotion for head louse infestation. Med Devices (Auckl). 2011;4:35–42.
Burgess IF, Burgess NA, Brunton ER. Tocopheryl acetate 20% spray for elimination of head louse infestation: a randomised controlled trial comparing with 1% permethrin creme rinse. BMC Pharmacol Toxicol. 2013;14:43.
Grieve KA, Altman PM, Rowe SJ, Staton JA, Oppenheim VMJ. A randomised, double-blind, comparative efficacy trial of three head lice treatment options: malathion, pyrethrins with piperonyl butoxide and MOOV Head Lice Solution. Aust. Pharmacist. 2007;26:738–43.
Koch E, Clark JM, Cohen B, Meinking TL, Ryan WG, Stevenson A, et al. Management of head louse infestations in the United States—a literature review. Ped Dermatol. 2016;33:466–72.
Gellatly KJ, Krim S, Palenchar DJ, Shepherd K, Yoon KS, Rhodes CJ, et al. Expansion of the knockdown resistance frequency map for human head lice (Phthiraptera: Pediculidae) in the United States using quantitative sequencing. J Med Entomol. 2016;53:653–9.
Acknowledgements
Funding
Sponsorship for this study and article processing charges were funded by Oystershell Laboratories, which is also the manufacturer of the investigational product and employer of the co-authors Frank Eertmans and Bart Rossel. The sponsor was involved in the study planning as well as in the decision to publish, but was not involved in data collection, data analysis, and data interpretation. All authors had full access to all data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Other Assistance
Oystershell Laboratories engaged a CRO in Florida (USA), specialized in clinical trials with pediculicides, to independently design and perform the study. Two centers were involved: Lice Source Services (Plantation) and Lice Cleanique (Miami). Co-author Elisabeth River was the Principal Investigator and Lidia Serrano was study monitor. We thank all subjects for participating in the clinical trial. Statistical analyses were performed by an independent, external consultant, Els Adriaens (Adriaens Consulting, Bellem, Belgium).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval for the version to be published.
Disclosures
Frank Eertmans is an employee of the company Oystershell, which is the developer of the test product and funder of this study. Bart Rossel is an employee of the company Oystershell, which is the developer of the test product and funder of this study. Elisabeth Rivera is a clinical investigator, associated with the two clinical trial centers, who performed the study. Lidia Serrano is a clinical investigator, associated with the two clinical trial centers, who performed the study. Els Adriaens has nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7364558.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Eertmans, F., Rossel, B., Serrano, L. et al. Efficacy and Safety of a Water-Based Head Lice Lotion: A Randomized, Controlled, Investigator-Blinded, Comparative, Bicentric Study. Dermatol Ther (Heidelb) 9, 143–157 (2019). https://doi.org/10.1007/s13555-018-0274-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-018-0274-x