Abstract
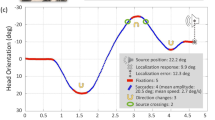
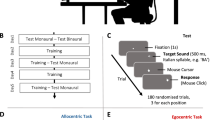
Exposure of humans to unusual spaces is effective to observe the adaptive strategy for an environment. Though adaptation to such spaces has been typically tested with vision, little has been examined about adaptation to left–right reversed audition, partially due to the apparatus for adaptation. Thus, it is unclear if the adaptive effects reach early auditory processing. Here, we constructed a left–right reversed stereophonic system using only wearable devices and asked two participants to wear it for 4 weeks. Every week, the magnetoencephalographic responses were measured under the selective reaction time task, where they immediately distinguished between sounds delivered to either the left or the right ear with the index finger on the compatible or incompatible side. The constructed system showed high performance in sound localization and achieved gradual reduction of a feeling of strangeness. The N1m intensities for the response-compatible sounds tended to be larger than those for the response-incompatible sounds until the third week but decreased on the fourth week, which correlated with the initially shorter and longer reaction times for the compatible and incompatible conditions, respectively. In the second week, disruption of the auditory-motor connectivity was observed with the largest N1m intensities and the longest reaction times, irrespective of compatibility. In conclusion, we successfully produced a high-quality space of left–right reversed audition using our system. The results suggest that a 4-week exposure to the reversed audition causes optimization of the auditory-motor coordination according to the new rule, which eventually results in the modulation of early auditory processing.







Similar content being viewed by others
References
Sugita Y. Visual evoked potentials of adaptation to left–right reversed vision. Percept Mot Skills. 1994;79(2):1047–54.
Sekiyama K, Miyauchi S, Imaruoka T, Egusa H, Tashiro T. Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature. 2000;407(6802):374–7.
Miyauchi S, Egusa H, Amagase M, Sekiyama K, Imaruoka T, Tashiro T. Adaptation to left–right reversed vision rapidly activates ipsilateral visual cortex in humans. J Physiol Paris. 2004;98(1–3):207–19.
Sekiyama K, Hashimoto K, Sugita Y. Visuo-somatosensory reorganization in perceptual adaptation to reversed vision. Acta Psychol (Amst). 2012;141(2):231–42.
Stratton GM. Some preliminary experiments on vision without inversion of the retinal image. Psychol Rev. 1896;3(6):611–7.
Linden DE, Kallenbach U, Heinecke A, Singer W, Goebel R. The myth of upright vision. A psychophysical and functional imaging study of adaptation to inverting spectacles. Perception. 1999;28(4):469–81.
Young TP. Auditory localization with acoustical transposition of the ears. J Exp Psychol. 1928;11(6):399–429.
Willey CF, Inglis E, Pearce CH. Reversal of auditory localization. J Exp Psychol. 1937;20(2):114–30.
Hofman PM, Vlaming MS, Termeer PJ, Van Opstal AJ. A method to induce swapped binaural hearing. J Neurosci Methods. 2002;113(2):167–79.
Thompson SP. The pseudophone. Philos Mag Ser 5. 1879;8(50):385–90.
Welch RB. Perceptual modification: adapting to altered sensory environments. New York: Academic Press; 1978.
Ohtsubo H, Teshima T, Najamizo S. Effects of head movements on sound localization with an electronic pseudophone. Jpn Psychol Res. 1980;22(3):110–8.
Held R. Shifts in binaural localization after prolonged exposures to atypical combinations of stimuli. Am J Psychol. 1955;68(4):526–48.
Freedman SJ, Stampfer K. The effects of displaced ears on auditory localization. Technical Report AFOSR. 1964; 64-0938, p. 1–24.
Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32(1):35–42.
Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26(1):55–67.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis—I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis—II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207.
Aoyama A, Haruyama T, Kuriki S. Early auditory change detection implicitly facilitated by ignored concurrent visual change during a Braille reading task. J Integr Neurosci. 2013;12(3):385–99.
Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–38.
Barnett L, Seth AK. The MVGC multivariate Granger causality toolbox: a new approach to Granger-causal inference. J Neurosci Methods. 2014;223:50–68.
Green DM. Temporal auditory acuity. Psychol Rev. 1971;78(6):540–51.
Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25(6):465–76.
Tsunada J, Liu AS, Gold JI, Cohen YE. Causal contribution of primate auditory cortex to auditory perceptual decision-making. Nat Neurosci. 2016;19(1):135–42.
Ragot R, Fiori N. Mental processing during reactions toward and away from a stimulus: an ERP analysis of auditory congruence and S-R compatibility. Psychophysiology. 1994;31(5):439–46.
Nandrino JL, el Massioui F. Temporal localization of the response selection processing stage. Int J Psychophysiol. 1995;19(3):257–61.
Roswarski TE, Proctor RW. Auditory stimulus-response compatibility: is there a contribution of stimulus-hand correspondence? Psychol Res. 2000;63(2):148–58.
Maslovat D, Carlsen AN, Franks IM. Investigation of stimulus-response compatibility using a startling acoustic stimulus. Brain Cogn. 2012;78(1):1–6.
Endo H, Kizuka T, Masuda T, Takeda T. Automatic activation in the human primary motor cortex synchronized with movement preparation. Brain Res Cogn Brain Res. 1999;8(3):229–39.
Sharma A, Campbell J, Cardon G. Developmental and cross-modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children. Int J Psychophysiol. 2015;95(2):135–44.
Sandmann P, Plotz K, Hauthal N, de Vos M, Schönfeld R, Debener S. Rapid bilateral improvement in auditory cortex activity in postlingually deafened adults following cochlear implantation. Clin Neurophysiol. 2015;126(3):594–607.
Veniero D, Vossen A, Gross J, Thut G. Lasting EEG/MEG aftereffects of rhythmic transcranial brain stimulation: level of control over oscillatory network activity. Front Cell Neurosci. 2015. doi:10.3389/fncel.2015.00477.
Acknowledgements
This work was partially supported by a grant from JSPS KAKENHI Grant Number JP26730078. The authors thank Kazuhiro Shigeta and Takayuki Hoshino for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Aoyama, A., Kuriki, S. A wearable system for adaptation to left–right reversed audition tested in combination with magnetoencephalography. Biomed. Eng. Lett. 7, 205–213 (2017). https://doi.org/10.1007/s13534-017-0026-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-017-0026-3