Abstract
Background
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes mellitus (DM). It is characterized by hyperglycemia, metabolic acidosis, and ketonemia. Fortunately, drug-induced hyperglycemias are usually mild and not life-threatening. However, rarely some cases may present with ketoacidosis. In this case report, we aimed to present a brentuximab vedotin (BV) associated with DKA.
Case presentation
A 23-year-old Caucasian man presented with abdominal pain, nausea, and vomiting for 1–2 weeks. The patient had a previous diagnosis of Hodgkin’s lymphoma and primer hypothyroidism. He is using levothyroxine 150 μg per day and received BV treatment for Hodgkin lymphoma (HL) 10 days ago. No steroid treatment was administered for premedication before BV. Except for obesity, all system examinations are normal. There were no signs of any infection. Laboratory data revealed hyperglycemia, metabolic acidosis, and ketonemia. The patient was admitted to the service with a diagnosis of DKA. After the patient was admitted to our clinic, insulin treatment and hydration started immediately. Despite the insulin infusion reaching 1700 units per day, the patient’s diabetic ketoacidosis extended to 1 week. Anti-insulin, anti-glutamic acid decarboxylase, and islet cell autoantibodies were negative, which were checked to exclude type 1 DM. Fasting C-peptide was 28 ng/mL (normal range, 0.9–7.1 ng/mL). With all these, the diabetic ketoacidosis status of the patient was evaluated as a BV side effect.
Conclusion
This patient is a rare case of BV-associated DKA. It is very important to know this relationship since BV treatment has turned into a standard treatment for relapsed Hodgkin lymphoma. Our case highlights that this diagnosis should be kept in mind as a complication of each dose of BV administration.
Similar content being viewed by others
Background
Diabetic ketoacidosis (DKA) is one of the most important emergencies of diabetes mellitus (DM). It is characterized by hyperglycemia, metabolic acidosis, and ketonemia. Although it is more common in type 1 DM, it can also be seen in all types of DM. DKA occurs due to absolute/relative insulin deficiency with or without increased counter-regulatory hormones and is often precipitated by external factors like acute major illnesses, trauma, dehydration or drugs [1].
Brentuximab vedotin (BV) is a drug used in Hodgkin lymphoma (HL) containing an anti-CD30 antibody linked to the anti-tubulin agent monomethyl auristatin [2]. Although generally well-tolerated, there are also dose-limiting side effects. Hyperglycemia is one of them, especially in high-dose therapy [3]. In this article, we presented a case of diabetic ketoacidosis after BV treatment.
Case report
A 23-year-old Caucasian man presented emergency room with abdominal pain, nausea, and vomiting. The pain was dull, continuous, and localized to the periumbilical region, without any bowel or bladder complaints. He had a previous diagnosis of HL for 4 years, primary hypothyroidism for ten years and obesity for 5 years. He was using levothyroxine 150 μg per day and received a fourth BV infusion for HL 10 days ago. He had no history of alcohol and smoking. The patient was not working.
On a physical examination, his weight was 135 kg, height 1.78 m, and body mass index 42.7 kg/m2. His vital signs were normal besides sinus tachycardia (194/min). There was no high fever and no focus of infection was detected. Throat, respiratory tract, and abdominal examinations were normal. The perianal region was normal. No acanthosis nigricans, lipodystrophy, or signs of cortisol excess existed. His skin and mouth were dry due to dehydration.
Laboratory tests showed hyperglycemia, acidosis, and ketonemia at emergency admission [glucose: 306 mg/dL (normal range, 74–106 mg/dL), ph: 7.25 (normal range, HCO3: 14.9 mmol/L (normal range, 22–26 mmol/L), serum ketone level: 6.2 mmoL/L (normal range, 0.02–0.27 mmol/L)]. He internalized to the endocrine clinic with a DKA diagnosis. Laboratory tests performed in our clinic are in Table 1. There was no pathology on the chest X-ray and the COVID-19 polymerase chain reaction was negative.
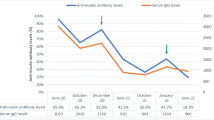
In the detailed history, the patient did not have a covid infection and had the COVID-19 mRNA vaccine 6 months ago. He has no history of DKA and HHS but he had hyperglycemia 2 years ago after his first dose of BV therapy. The patient’s hyperglycemia at that time had started at a similar timing to now, 1 week after BV treatment. In the patient with polyuria polydipsia, HbA1c: 6.8% and fasting blood glucose: 206 mg/dl were found, respectively. The ketone was not detected in the complete urinalysis. Linagliptin was prescribed to the patient with a DM diagnosis whose general condition was good. Before 1 month of the first BV treatment, the patient’s glycemic course was normal and Hba1c was 5.3%. The patient was also obese and had a body mass index of 42.7 at that time. The patient who used linagliptin for 1 month was normoglycemic during home follow-ups, so the drug was discontinued and HbA1c was detected at 5.3%, 3 months later (Fig. 1).
The treatment of DKA was initiated as, insulin infusion (0.15 IU/kg/hour), 0.9% NaCl (1 L/h) therapy, and potassium replacement (20 mEq each liter of iv fluid) immediately. The daily insulin need of the patient, whose DKA did not improve despite 1 week of appropriate treatment, reached 1700 units/per 24 h. DKA resolved on the 8th day of hospitalization and insulin infusion continued for three more days due to the intense insulin need. When DKA resolved, metformin was added to the treatment. After the addition of metformin, the patient’s insulin requirement gradually decreased (Fig. 2). After insulin infusion stopped, basal-bolus insulin therapy started at 0.5 IU/kg/day. Euglycemia was achieved with dose titration. The patient was discharged on the 12th hospital day. The patient was discharged with basal-bolus insulin therapy. The dose requirement gradually decreased, and after 2 months, insulin and metformin treatment were completely discontinued. At the end of 3 months without treatment, the patient’s current Hba1c is 5.3%. He continues to be followed up.
Discussion
Despite the widespread use of BV and its use as a first-line treatment for relapsed HL, the reported case of BV-associated DKA is extremely rare. Awareness of the hyperglycemia side effect of BV would be effective in early diagnosis and reduction of mortality, especially for DKA.
BV is an antibody-drug conjugate directed against the CD-30 antigen expressed on classical HL and anaplastic large cell lymphoma. It is leading to subsequent internalization of the anti-tubulin agent and cell death [4]. In the BV phase I clinical trial, treatment doses ranged from 0.1 to 3.6 mg/kg. With these doses, the most common adverse effects were fatigue (36%), fever (33%), nausea (22%), neutropenia (22%), and peripheral neuropathy (22%), followed by headache, vomiting, back pain, anemia, and alopecia. One patient had grade 3 hyperglycemia at a dose of 2.7 milligrams per kilogram, while patients treated with 1.8 mg/kg did not [5]. In our case, the patient’s treatment dose was 1.8 mg/kg. Perhaps this dose was as much as the maximum total dose given in the 2.7 mg/kg treatment arm in the phase 1 study due to patient obesity. A search of the U.S. Food and Drug Administration Adverse Events Reporting System showed 58 reported reactions of hyperglycemia with resulted in 10 patient death and 20 DKA with 6 death [6].
Drug-induced hyperglycemia is usually mild and reversible. Glucocorticoids, somatostatin analogs, diuretics, statins, and antipsychotics are well-known diabetogenic drugs [7, 8]. However, with the development of new immunotherapy agents and chemotherapy regimens recently, many new diabetogenic drugs have also been defined. Drugs may cause hyperglycemia with a variety of mechanisms, including alterations in insulin secretion and sensitivity, direct cytotoxic effects on pancreatic beta cells, and increases in glucose production [7]. We suspected DKA with severe insulin resistance due to diminution of peripheral insulin sensitivity in our case. He had hyperinsulinemia in his biochemical test done before insulin infusion treatment and also insulin requirement decreased rapidly after initiation of metformin therapy, suggesting insulin resistance. There was no finding suggestive of pancreatitis. Like many diabetogenic drugs, BV also causes temporary DM. Our patient had hyperglycemia after his first BV therapy 2 years ago and used linagliptin for 1 month. His weight has been stable for the last 6 years and he did not have DM before that despite obesity and metabolic syndrome.
Apart from our case, there is only one case report related to DKA after BV treatment. That patient also had human immunodeficiency virus infection and he died due to multiple organ failures. In that case, after the first dose of BV administration, the patient had a systemic cytokine release with extreme insulin resistance. Although IV insulin was titrated to > 600 units/h, blood glucose regulation could not be achieved [9]. The patient’s diagnosis of AIDS and cytokine storm at presentation may have played a role in the etiology of diabetic ketoacidosis in that case. Because the patient died, it could not be predicted whether the diabetes mellitus would be temporary or permanent. Since we had the chance of long-term follow-up with our patient, we were able to document the hyperglycemic periods that developed after both cycles and that these conditions were temporary, in addition to those presented in this case. No hyperglycemia was detected in another case report in which cytokines storm was described with BV [10]. The first case has class 2 obesity as in our case [9]. However, we do not know about the body mass index of another case[10]. The resulting IL-6 was 17.7 pg/mL (normal < 5.9 pg/mL) and there was no cytokine storm clinic in our patient. Although there are cases of type 1 DM reported in drug-induced hyperglycemias, we excluded this with negative antibodies and sufficient C-peptide levels in our patient. There is no reported type 1 DM associated with BV.
We think that the condition is related to brentuximab, as our patient developed hyperglycemia after both treatment cycles and regressed within a few months after the treatment. However, while the hyperglycemic state that occurred the first time was milder, we could not explain his application with diabetic ketoacidosis the second time. In both cases, the patient did not have an infection or systemic comorbidity. His weight and total treatment doses were the same in both treatments. Maybe this diabetic process was also related to an antibody that we do not know about and caused a more severe response in the body of the sensitized individual in the 2nd cycle.
Finally, BV is a drug that is increasingly used in hematologic malignancies today. Although the side effects are mostly tolerable, we should also be aware of life-threatening situations like DKA.
Conclusion
DKA due to BV therapy is a very rare condition. However, these cases may increase relatively due to the frequent use of BV. Since this situation may be temporary, it should be considered in the etiology of DM. These patients should be followed closely and should be reevaluated after BV therapy. Currently, treatment is the same as standard DM and DKA besides severe insulin resistance and high insulin need. However, as molecular studies are carried out to define the hyperglycemia mechanism, specific treatments for the mechanism can be developed.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism: Clinical and Experimental. 2016;65:507–21.
Ansell SM. Hodgkin Lymphoma: Diagnosis and Treatment. In: Hodgkin lymphoma: diagnosis and treatment. In: Mayo Clinic Proceedings. Elsevier Ltd; 2015. p. 1574–83.
Makita S, Maruyama D, Tobinai K. Safety and efficacy of brentuximab vedotin in the treatment of classic Hodgkin lymphoma. OncoTargets and Therapy. 2020;13:5993–6009. https://doi.org/10.2147/OTT.S193951.
Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clinical Cancer Research. 2011;17:6428–36. https://doi.org/10.1158/1078-0432.CCR-11-0488
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab Vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21. https://doi.org/10.1056/NEJMoa1002965
FDA Adverse Event Reporting System (FAERS) Public Dashboard | FDA. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed 2 Apr 2021.
Fathallah N, Slim R, Larif S, Hmouda H, Ben SC. Drug-induced hyperglycaemia and diabetes. Drug Safety. 2015;38:1153–68. https://doi.org/10.1007/s40264-015-0339-z.
Jain V, Patel RK, Kapadia Z, Galiveeti S, Banerji M, Hope L. Drugs and hyperglycemia: a practical guide. Maturitas. 2017;104:80–3.
Chiang JM, Lai AR, Anderson M, Rushakoff RJ. Severe insulin resistance with diabetic ketoacidosis after Brentuximab treatment. AACE Clin Case Reports. 2020;6:e98–100.
Alig SK, Dreyling M, Seppi B, Aulinger B, Witkowski L, Rieger CT. Severe cytokine release syndrome after the first dose of Brentuximab Vedotin in a patient with relapsed systemic anaplastic large cell lymphoma (sALCL): a case report and review of the literature. Eur J Haematol. 2015;94:554–7. https://doi.org/10.1111/ejh.12396.
Acknowledgments
None.
Funding
There was no funding for this study by any means.
Author information
Authors and Affiliations
Contributions
DK, MS, and AS performed concept, design, and data acquisition. DK, MS, and EG performed clinical studies, literature search, and manuscript review. DK, MS, BÇ, and ZC performed manuscript preparation, and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
A written consent was signed by the patient for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Köksalan, D., Sözen, M., Selek, A. et al. Brentuximab vedotin-associated diabetic ketoacidosis: a case report. Int J Diabetes Dev Ctries 43, 120–124 (2023). https://doi.org/10.1007/s13410-022-01116-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01116-w