Abstract
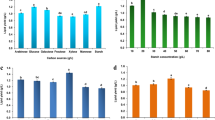
Oleaginous yeast lipids have myriad of industrial applications that are gaining significant interest owing to shorter incubation, ability to use broad spectrum substrates, and quality lipids. However, the lipid content produced is low and need to enhance by optimization of varied parameters. In the present study, crude glycerol a by-product of biodiesel industry was supplemented to Trichosporon shinodae for lipid accumulation using central composite design (CCD) of response surface methodology (RSM). The developed quadratic model was found to be significant with the R2 value of 95.20% and adj. R2 value of 91.97%. An optimal lipid content of 49.85 ± 0.8% (w/w) was obtained using T. shinodae with 6.2% (v/v) inoculum volume, pH 3.6, C/N ratio 105, 1.52 (g/L) of MgSO4, and 4.55 mM FeSO4 in 120.72 h at 30 °C. Lipid composition from T. shinodae depicted the presence of linoleic acid (C18:2), oleic acid (C18:1), stearic acid (C18:0), palmitic acid (C18:0), myristic acid (C14:0), and lauric acids (C12:0), respectively. T. shinodae lipids have 61.1% (w/w) saturated fatty acids and unsaturated fatty acids proportion accord to 38.9% (w/w). Lipid composition of T. shinodae indicates that these lipids were suitable for synthesis of high value products like fuel additives, surfactants, detergents, and cleaning applications.
Graphical abstract



Similar content being viewed by others
References
Banerjee R, Kumar SPJ, Mehendale N, Sevda S, Garlapati VK (2019) Intervention of microfluidics in biofuel and bioenergy sectors: technological considerations and future prospects. Renew Sustain Energy Rev 101:548–558. https://doi.org/10.1016/j.rser.2018.11.040
Gujjala LKS, Kumar SPJ, Talukdar B, Dash A, Kumar S, Sherpa KCH, Banerjee R (2017) Biodiesel from oleaginous microbes: opportunities and challenges. Biofuels 1: 1–15. https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/17597269.2017.1402587
Cho HU, Park JM (2018) Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour Technol 256:502–508. https://doi.org/10.1016/j.biortech.2018.02.010
Kumar SPJ, Banerjee R (2019) Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: a greener and scalable process. Ultrason Sonochem 52:25–32. https://doi.org/10.1016/j.ultsonch.2018.08.003
Kumar SPJ, Kumar NS, Chintagunta AD (2020) Bioethanol production from cereal crops and lignocelluloses rich agro-residues: prospects and challenges. SN Appl Sci 10:1–1. https://doi.org/10.1007/s42452-020-03471-x
Vlysidis A, Binns M, Webb C, Theodoropoulos C (2011) A techno-economic analysis of biodiesel biorefineries: assessment of integrated designs for the co-production of fuels and chemicals. Energy 36:4671–4683. https://doi.org/10.1016/j.energy.2011.04.046
Varrone C, Liberatore R, Crescenzi T, Izzo G, Wang A (2013) The valorization of glycerol: economic assessment of an innovative process for the bioconversion of crude glycerol into ethanol and hydrogen. Appl Energy 105:349–357. https://doi.org/10.1016/j.apenergy.2013.01.015
Kumari A, Mahapatra P, Garlapati VK, Banerjee R (2009) Enzymatic transesterification of Jatropha oil. Biotechnol Biofuels 2:1. https://doi.org/10.1186/1754-6834-2-1
Garlapati VK, Kant R, Kumari A, Mahapatra P, Das P, Banerjee R (2013) Lipase mediated transesterification of Simarouba glauca oil: a new feedstock for biodiesel production. Sustain Chem Process 1:11. https://doi.org/10.1186/2043-7129-1-11
Garlapati VK, Mohapatra SB, Mohanty RC, Das P (2021) Transesterified Olax scandens oil as a bio-additive: production and diesel engine performance studies. Tribol Int 153:106653. https://doi.org/10.1016/j.triboint.2020.106653
Banerjee R, Chintagunta A, Ray S (2017) A cleaner and eco-friendly bioprocess for enhancing reducing sugar production from pineapple leaf waste. J Clean Prod 149:387–395. https://doi.org/10.1016/j.jclepro.2017.02.088
Talebian-Kiakalaieh A, Amin NAS, Mazaheri H (2013) A review on novel processes of biodiesel production from waste cooking oil. Appl Energy 104:683–710. https://doi.org/10.1016/j.apenergy.2012.11.061
Souza KST, Ramos CL, Schwan RF, Dias DR (2016) Lipid production by yeasts grown on crude glycerol from biodiesel industry. Prep Biochem Biotechnol 1–7. https://doi.org/10.1080/10826068.2016.1244689
Patel A, Arora N, Mehtani J, Pruthi V, Pruthi PA (2017) Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew Sustain Energy Rev 77:604–616. https://doi.org/10.1016/j.rser.2017.04.016
Wu H, Li Y, Chen L, Zong M (2011) Production of microbial oil with high oleic acid content by Trichosporon capitatum. Appl Energy 88:138–142. https://doi.org/10.1016/j.apenergy.2010.07.028
Sharma D, Garlapati VK, Goel G (2016) Bioprocessing of wheat bran for the production of lignocellulolytic enzyme cocktail by Cotylidia pannosa under submerged conditions. Bioengineered 7(2):88–97. https://doi.org/10.1080/21655979.2016.1160190
Bezerraa MA, Santelli RE, Oliveiraa EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Chauhan M, Chauhan RS, Garlapati VK (2013) Modelling and optimization studies on a novel lipase production by Staphylococcus arlettae through submerged fermentation. Enzyme Res 2013: Article ID 353954, 1–8. https://doi.org/10.1155/2013/353954
Kumar SPJ, Banerjee R (2013) Optimization of lipid enriched biomass production from oleaginous fungus using response surface methodology. Indian J Exp Biol 51: 979–983. http://nopr.niscair.res.in/handle/123456789/23466
Gorte O, Kugel M, Ochsenreither K (2020) Optimization of carbon source efficiency for lipid production with the oleaginous yeast Saitozyma podzolica DSM 27192 applying automated continuous feeding. Biotechnol Biofuels 13:181. https://doi.org/10.1186/s13068-020-01824-7
Awad D, Bohnen F, Mehlmer N, Brueck T (2019) Multi-factorial-guided media optimization for enhanced biomass and lipid formation by the oleaginous yeast Cutaneotrichosporon oleaginosus. Front Bioeng Biotechnol 26(7):54. https://doi.org/10.3389/fbioe.2019.0005426
Jacquet N, Haubruge E, Richel A (2015) Production of biofuels and biomolecules in the framework of circular economy: a regional case study. Waste Manage Res 33:1121–1126. https://doi.org/10.1177/0734242X15613154
Kumar SPJ, Gujjala LKS, Dash A, Talukdar B, Banerjee R (2017) Biodiesel production from lignocellulosic biomass using oleaginous microbes. In: Kuila A, Sharma V (eds) Lignocellulosic biomass production and industrial applications. Wiley, New York, pp 65–92
Salakkam A, Webb C (2015) The inhibition effect of methanol as a component of crude glycerol on the growth rate of Cupriavidus necator and other microorganisms. Biochem Eng J 98:84–90. https://doi.org/10.1016/j.bej.2015.02.024
Uprety BK, Dalli SS, Rakshit SK (2017) Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Convers Manage 135:117–128. https://doi.org/10.1016/j.enconman.2016.12.071
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Garlapati VK, Banerjee R (2010) Optimization of lipase production using differential evolution. Biotechnol Bioproc E 15:254–260. https://doi.org/10.1007/s12257-009-0163-3
Garlapati VK, Kumari A, Mahapatra P, Banerjee R (2013) Modeling, simulation and kinetic studies of solvent-free biosynthesis of benzyl acetate. J Chem 2013: Article ID 451652, 1–9, doi: https://doi.org/10.1155/2013/451652.
Forfang K, Zimmermann B, Kosa G, Kohler A, Shapaval V (2017) FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoS ONE 12:e0170611. https://doi.org/10.1371/journal.pone.0170611
Sorate KA, Bhale PV (2015) Biodiesel properties and automotive system compatibility issues. Renew Sustain Energy Rev 41:777–798. https://doi.org/10.1016/j.rser.2014.08.079
Polburee P, Yongmanitchai W, Honda K, Ohashi T, Yoshida T, Fujiyama K, Limtong S (2016) Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Biochem Eng J 112:208–218. https://doi.org/10.1016/j.bej.2016.04.024
Jernejc K, Legisa M (2002) The influence of metal ions on malic enzyme activity and lipid synthesis in Aspergillus niger. FEMS Microbiol Lett 217:185–190. https://doi.org/10.1111/j.1574-6968.2002.tb11473.x
Saenge C, Cherisilp B, Suksaroge TT, Bourtoom T (2011) Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol Bioprocess Eng 16:23–33. https://doi.org/10.1007/s12257-010-0083-2
Immelman M, Du Preez JC, Kilian SG (1997) Effect of C: N ratio on gamma-linolenic acid production by Mucor circinelloides grown on acetic acid. Syst Appl Microbiol 20:158–164. https://doi.org/10.1016/S0723-2020(97)80061-6
Man L, Behera SK, Park HS (2010) Optimization of operational parameters for ethanol production from Korean food waste leachate. Int J Environ Sci Tech 7:157–164. https://doi.org/10.1007/BF03326127
Xu J, Zhao X, Wang W, Du W, Liu D (2012) Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem Eng J 65:30–36. https://doi.org/10.1016/j.bej.2012.04.003
Saenge C, Cherisilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218. https://doi.org/10.1016/j.procbio.2010.08.009
Zhang J, Bilal M, Liu S, Zhang J, Lu H, Luo H, Luo C, Shi H, Hafiz M, Iqbal N, Zhao Y (2019) Sustainable biotransformation of oleic acid to 10-hydroxystearic acid by a recombinant oleate hydratase from Lactococcus garvieae. Proces 7:326. https://doi.org/10.3390/pr7060326
Knothe G, Bagby MO, Ryan TW (1998) Precombustion of fatty acids and esters of biodiesel. A possible explanation for differing cetane numbers. J Amer Oil Chem Soc 75:1007–1013. https://doi.org/10.1007/s11746-998-0279-1
Dunn RO, Knothe GH (2003) Oxidative stability of biodiesel/jet fuel blends by oil stability index (osi) analysis. J Am Oil Chem Soc 80:1047–1048. https://doi.org/10.1007/s11746-003-0818-6
Rabelo SN, Ferraz VP, Oliveira LS, Franca AS (2015) FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int J Environ Sci Dev 6:964. https://doi.org/10.7763/IJESD.2015.V6.730
Soares IP, Rezende TF, Silva RC, Castro EV, Fortes IC (2008) Multivariate calibration by variable selection for blends of raw soybean oil/biodiesel from different sources using Fourier transform infrared spectroscopy (FTIR) spectra data. Energy Fuels 22:2079–2083. https://doi.org/10.1021/ef700531n
Dubé MA, Zheng S, McLean DD, Kates M (2004) A comparison of attenuated total reflectance-FTIR spectroscopy and GPC for monitoring biodiesel production. J Am Oil Chem Soc 81:599–603. https://doi.org/10.1007/s11746-006-0948-x
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507. https://doi.org/10.1016/j.biortech.2010.01.065
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Jaramillo-Jacob ADR (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111. https://doi.org/10.1016/j.fuel.2011.06.070
Datta A, Mandal BK (2016) A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew Sustain Energy Rev 57:799–821. https://doi.org/10.1016/j.rser.2015.12.170
Gopinath A, Puhan S, Nagarajan G (2009) Theoretical modeling of iodine value and saponification value of biodiesel fuels from their fatty acid composition. Renew Energy 34:1806–1811. https://doi.org/10.1016/j.renene.2008.11.023
Nouri H, Moghimi H, Rad MN, Ostovar M, Mehr SS, Ghanaatian F, Talebi AF (2019) Enhanced growth and lipid production in oleaginous fungus, Sarocladium kiliense ADH17: study on fatty acid profiling and prediction of biodiesel properties. Renew Energy 135:10–20. https://doi.org/10.1016/j.renene.2018.11.104
Vicente G, Martınez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305. https://doi.org/10.1016/j.biortech.2003.08.014
Toscano G, Riva G, Foppa Pedretti E, Duca D (2012) Vegetable oil and fat viscosity forecast models based on iodine number and saponification number. Biomass Bioenerg 46:511–516. https://doi.org/10.1016/j.biombioe.2012.07.009
Tao BY (2007) Industrial applications for plant oils and lipids. In: Shang-Tian Y (ed) Bioprocessing for value-added products from renewable resources. Elsevier Science, USA, pp 611–627
Acknowledgements
Authors are grateful to the Director, Indian Institute of Technology, Kharagpur for all the support rendered in the completion of the work.
Author information
Authors and Affiliations
Contributions
SPJK has conducted the experiments, analyzed the data, and drafted the paper. GVK analysed the RSM data and drafted the paper. RB conceived the research work, analysed, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeevan Kumar, S.P., Kumar Garlapati, V., Lohit Kumar Srinivas, G. et al. Bioconversion of waste glycerol for enhanced lipid accumulation in Trichosporon shinodae. Biomass Conv. Bioref. 13, 15401–15412 (2023). https://doi.org/10.1007/s13399-021-01799-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01799-x