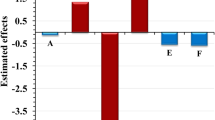
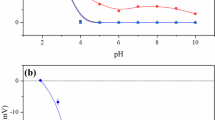
Abstract
Comprehensive processing of Japonochytrium sp., one of marine fungi considered as a perspective source of functional lipids, was tested as an example of circular economy. Residual biomass after extraction of lipids (omega-3 polyunsaturated fatty acids and triacylglycerols) was magnetically modified using methanol suspension of microwave-synthesized magnetic iron oxides and tested as a potential biosorbent for triphenylmethane dye, crystal violet, used in aquaculture. Employing a batch experimental setup, influence of pH value (3–9), incubation time (0–270 min), initial dye concentration (400–1000 mg/L) of crystal violet, and temperature (282.15–313.15 K) on adsorption efficacy was studied. Adsorption equilibrium data were analyzed and fitted to the Langmuir, Freundlich, and Sips isotherm models. The monolayer maximum adsorption capacity of magnetically responsive spent biomass was found to be 329.22 mg/g at 294.15 K. The adsorption process agreed best with the pseudo-second-order kinetic model, and thermodynamic studies showed an endothermic nature of adsorption.



Similar content being viewed by others
References
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal - a review. J Environ Manage 90(8):2313–2342
Carmen Z, Daniela S (2012) Textile organic dyes - characteristics, polluting effects and separation/elimination procedures from industrial effluents - a critical overview. In: Puzyn T, Mostrag-Szlichtyng A (eds) Organic pollutants ten years after the Stockholm convention - environmental and analytical update. InTech, Rijeka, pp 55–86
Baldikova E, Politi D, Maderova Z, Pospiskova K, Sidiras D, Safarikova M, Safarik I (2016) Utilization of magnetically responsive cereal by-product for organic dye removal. J Sci Food Agric 96(6):2204–2214
Bergwerff AA, Scherpenisse P (2003) Determination of residues of malachite green in aquatic animals. J Chromatogr B 788(2):351–359
Andersen WC, Turnipseed SB, Karbiwnyk CM, Lee RH, Clark SB, Rowe WD, Madson MR, Miller KE (2009) Multiresidue method for the triphenylmethane dyes in fish: malachite green, crystal (gentian) violet, and brilliant green. Anal Chim Acta 637(1–2):279–289
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255
Zhang J, Li Y, Zhang C, Jing Y (2008) Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J Hazard Mater 150(3):774–782
Adeyemo AA, Adeoye IO, Bello OS (2017) Adsorption of dyes using different types of clay: a review. Appl Water Sci 7(2):543–568
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97(9):1061–1085
Suba V, Rathika G (2016) Novel adsorbents for the removal of dyes and metals from aqueous solution-a review. J Adv Phys 5(4):277–294
Sarat Chandra T, Mudliar SN, Vidyashankar S, Mukherji S, Sarada R, Krishnamurthi K, Chauhan VS (2015) Defatted algal biomass as a non-conventional low-cost adsorbent: surface characterization and methylene blue adsorption characteristics. Bioresour Technol 184:395–404
Deniz F, Kepekci RA (2016) Equilibrium and kinetic studies of azo dye molecules biosorption on phycocyanin-extracted residual biomass of microalga Spirulina platensis. Desalin Water Treat 57(26):12257–12263
Vilar VJP, Botelho CMS, Boaventura RAR (2007) Chromium and zinc uptake by algae Gelidium and agar extraction algal waste: kinetics and equilibrium. J Hazard Mater 149(3):643–649
Vilar VJP, Botelho CMS, Boaventura RAR (2008) Copper removal by algae Gelidium, agar extraction algal waste and granulated algal waste: kinetics and equilibrium. Bioresour Technol 99(4):750–762
Bulgariu D, Bulgariu L (2012) Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour Technol 103(1):489–493
Inoue K, Gurung M, Adhikari BB, Alam S, Kawakita H, Ohto K, Kurata M, Atsumi K (2014) Adsorptive removal of cesium using bio fuel extraction microalgal waste. J Hazard Mater 271:196–201
Barrow CJ (2010) Marine nutraceuticals: glucosamine and omega-3 fatty acids. New trends for established ingredients. Agro Food Ind Hi Tech 21(2):36–41
Chang KJL, Paul H, Nichols PD, Koutoulis A, Blackburn SI (2015) Australian thraustochytrids: potential production of dietary long-chain omega-3 oils using crude glycerol. J Funct Food 19:810–820
Gupta A, Barrow CJ, Puri M (2012) Omega-3 biotechnology: thraustochytrids as a novel source of omega-3 oils. Biotechnol Adv 30(6):1733–1745
Dick MW (2001) Straminipilous fungi: systematics of the Peronosporomycetes including accounts of the marine straminipilous protists, the plasmodiophorids and similar organisms. Springer Netherlands, Dordrecht
Humhal T, Kastanek P, Jezkova Z, Cadkova A, Kohoutkova J, Branyik T (2017) Use of saline waste water from demineralization of cheese whey for cultivation of Schizochytrium limacinum PA-968 and Japonochytrium marinum AN-4. Bioprocess Biosyst Eng 40(3):395–402
Rouskova M, Maleterova Y, Hurkova K, Kastanek P, Hanika J, Solcova O (2017) Fatty acids profile analysis in fresh suspension of Japonochytrium sp. In: Proceedings of the 5th International Conference on Chemical Technology, 10–12. April 2017, Mikulov, Czech Republic, pp. 78–82
Safarik I, Baldikova E, Pospiskova K, Safarikova M (2016) Magnetic modification of diamagnetic agglomerate forming powder materials. Particuology 29:169–171
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(3):681–689
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89(2):31–60
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54(7):1981–1985
Daneshvar E, Kousha M, Jokar M, Koutahzadeh N, Guibal E (2012) Acidic dye biosorption onto marine brown macroalgae: isotherms, kinetic and thermodynamic studies. Chem Eng J 204:225–234
Bellisola G, Sorio C (2012) Infrared spectroscopy and microscopy in cancer research and diagnosis. Am J Cancer Res 2(1):1–21
Cheriaa J, Khaireddine M, Rouabhia M, Bakhrouf A (2012) Removal of triphenylmethane dyes by bacterial consortium. Sci World J 2012:1–9. https://doi.org/10.1100/2012/512454
Kumara N, Hamdan N, Petra MI, Tennakoon KU, Ekanayake P (2014) Equilibrium isotherm studies of adsorption of pigments extracted from Kuduk-kuduk (Melastoma malabathricum L.) pulp onto TiO2 nanoparticles. J Chem. https://doi.org/10.1155/2014/468975
Saeed A, Sharif M, Iqbal M (2010) Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J Hazard Mater 179:564–572
Pourjavadi A, Nazari M, Hosseini SH (2015) Synthesis of magnetic graphene oxide-containing nanocomposite hydrogels for adsorption of crystal violet from aqueous solution. RSC Adv 5:32263–32271
Sun P, Hui C, Azim Khan R, Du J, Zhang Q, Zhao Y-H (2015) Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci Rep (UK) 5:12638
Safarik I, Angelova R, Baldikova E, Pospiskova K, Safarikova M (2017) Leptothrix sp. sheaths modified with iron oxide particles: magnetically responsive, high aspect ratio functional material. Mater Sci Eng C 71:1342–1346
Jerold M, Vasantharaj K, Joseph D, Sivasubramanian V (2017) Fabrication of hybrid biosorbent nanoscale zero-valent iron-Sargassum swartzii biocomposite for the removal of crystal violet from aqueous solution. Int J Phytoremediation 19:214–224
Angelova R, Baldikova E, Pospiskova K, Maderova Z, Safarikova M, Safarik I (2016) Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal. J Clean Prod 137:189–194
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362
Madrakian T, Afkhami A, Ahmadi M (2012) Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim Acta A 99:102–109
Sahraei R, Sekhavat Pour Z, Ghaemy M (2017) Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: removal of heavy metals and dyes from water. J Clean Prod 142:2973–2984
Tahir N, Bhatti HN, Iqbal M, Noreen S (2017) Biopolymers composites with peanut hull waste biomass and application for crystal violet adsorption. Int J Biol Macromol 94:210–220
Sun P, Hui C, Khan RA, Guo X, Yang S, Zhao Y (2017) Mechanistic links between magnetic nanoparticles and recovery potential and enhanced capacity for crystal violet of nanoparticles-coated kaolin. J Clean Prod 164:695–702
Pourjavadi A, Hosseini SH, Seidi F, Soleyman R (2013) Magnetic removal of crystal violet from aqueous solutions using polysaccharide-based magnetic nanocomposite hydrogels. Polym Int 62:1038–1044
Safarik I, Horska K, Svobodova B, Safarikova M (2012) Magnetically modified spent coffee grounds for dyes removal. Eur Food Res Technol 234:345–350
Alizadeh N, Shariati S, Besharati N (2017) Adsorption of crystal violet and methylene blue on Azolla and fig leaves modified with magnetite iron oxide nanoparticles. Int J Environ Res 11:197–206
Safarik I, Lunackova P, Mosiniewicz-Szablewska E, Weyda F, Safarikova M (2007) Adsorption of water-soluble organic dyes on ferrofluid-modified sawdust. Holzforschung 61:247–253
Kanwal A, Bhatti HN, Iqbal M, Noreen S (2017) Basic dye adsorption onto clay/MnFe2O4 composite: a mechanistic study. Water Environ Res 89:301–311
Hamidzadeh S, Torabbeigi M, Shahtaheri SJ (2015) Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J Environ Health Sci Eng 13:8
Safarikova M, Pona BMR, Mosiniewicz-Szablewska E, Weyda F, Safarik I (2008) Dye adsorption on magnetically modified Chlorella vulgaris cells. Fresenius Environ Bull 17:486–492
Safarik I, Rego LFT, Borovska M, Mosiniewicz-Szablewska E, Weyda F, Safarikova M (2007) New magnetically responsive yeast-based biosorbent for the efficient removal of water-soluble dyes. Enzym Microb Technol 40:1551–1556
Salehi I, Shirani M, Semnani A, Hassani M, Habibollahi S (2016) Comparative study between response surface methodology and artificial neural network for adsorption of crystal violet on magnetic activated carbon. Arab J Sci Eng 41:2611–2621
Safarik I, Baldikova E, Prochazkova J, Safarikova M, Pospiskova K (2018) Magnetically modified agricultural and food waste: preparation and application. J Agric Food Chem 66:2538–2552
Badescu IU, Bulgariu D, Ahmad I, Bulgariu L (2018) Valorisation possibilities of exhausted biosorbents loaded with metal ions – a review. J Environ Manag 224:288–297
Funding
The research was supported by the projects LTC17020 and LO1305 (Ministry of Education, Youth and Sports of the Czech Republic) and by the ERDF/ESF project “New Composite Materials for Environmental Applications” (No. CZ.02.1.01/0.0/0.0/17_048/0007399).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 57 kb)
Rights and permissions
About this article
Cite this article
Baldikova, E., Mullerova, S., Prochazkova, J. et al. Use of waste Japonochytrium sp. biomass after lipid extraction as an efficient adsorbent for triphenylmethane dye applied in aquaculture. Biomass Conv. Bioref. 9, 479–488 (2019). https://doi.org/10.1007/s13399-018-0362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0362-2