Abstract
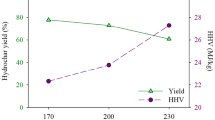
Corncob (CC) was converted to renewable fuel resource by hydrothermal carbonization (HTC). HTC was performed by varying process temperature (160–200 °C), residence time (1–3 h), and biomass to water ratio (BTW) (1:5 to 1:15). The properties of hydrochar were significantly enhanced where the fixed carbon and carbon content of hydrochar increased at about 24.9 and 83.7% from original contents in CC, respectively. The calorific values and yield of hydrochar were between 19.3–23.5 MJ/kg and 50.1–58.6%. The optimal condition for the production of hydrochar as solid fuel was determined at 200 °C, 3 h residence time, and BTW of 1:5 with maximum energy yield of 68.74%. In addition, hydrothermal liquid was characterized where volatile fatty acid, furfural, furfuryl alcohol, and hydroxymethylfurfural were the most abundant compositions with their highest yields of 17.3, 11.5, 7.9, and 5.1%, respectively. Process temperature was the most influencing variable on product properties and characteristics. The results suggested that corncob has high potential as a source for solid fuel and valuable platform chemicals.






Similar content being viewed by others
References
Benavente V, Calabuig E, Fullana A (2015) Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J Anal Appl Pyrolysis 113:89–98
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Emmerich K-H (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1):71–106. doi:10.4155/bfs.10.81
Acharya B, Dutta A, Minaret J (2015) Review on comparative study of dry and wet torrefaction. Sustain Energy Technol Assess 12:26–37
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4:160–177
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Kang S, Ye J, Zhang Y et al (2013) Preparation of biomass hydrochar derived sulfonated catalysts and their catalytic effects for 5-hydroxymethylfurfural production. RSC Adv 3:7360–7366
Hu B, Wang K, Wu L et al (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22:813–828
Kruse A, Funke A, Titirici M-M (2013) Hydrothermal conversion of biomass to fuels and energetic materials. Curr Opin Chem Biol 17:515–521
Basso D, Patuzzi F, Castello D et al (2016) Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag 47(Part A):114–121
Guo S, Dong X, Wu T et al (2015) Characteristic evolution of hydrochar from hydrothermal carbonization of corn stalk. J Anal Appl Pyrolysis 116:1–9
Álvarez-Murillo A, Román S, Ledesma B et al (2015) Study of variables in energy densification of olive stone by hydrothermal carbonization. J Anal Appl Pyrolysis 113:307–314
Gao P, Zhou Y, Meng F et al (2016) Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 97:238–245
Kongpanya J, Hussaro K, Teekasap S (2014) Influence of reaction temperature and reaction time on product from hydrothermal treatment of biomass residue. Am J Environ Sci 10:324–335
Gao Y, Wang X, Wang J et al (2013) Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 58:376–383
Ghanim BM, Pandey DS, Kwapinski W et al (2016) Hydrothermal carbonisation of poultry litter: effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour Technol 216:373–380
Sabio E, Álvarez-Murillo A, Román S et al (2016) Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: influence of the processing variables. Waste Manag 47(Part A):122–132
Tekin K, Akalin MK, Karagöz S (2016) The effects of water tolerant Lewis acids on the hydrothermal liquefaction of lignocellulosic biomass. J Energy Inst 89:627–635
Kim D, Yoshikawa K, Park K (2015) Characteristics of biochar obtained by hydrothermal carbonization of cellulose for renewable energy. Energies 8:12412
Erlach B, Harder B, Tsatsaronis G (2012) Combined hydrothermal carbonization and gasification of biomass with carbon capture. Energy 45:329–338
Heilmann SM, Davis HT, Jader LR et al (2010) Hydrothermal carbonization of microalgae. Biomass Bioenergy 34:875–882
Román S, Nabais JMV, Laginhas C et al (2012) Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process Technol 103:78–83
Centre for Agricultural Information (2015) Agricultural production data http://www.oae.go.th/ewt_news.php?nid=13577. Accessed 30 April 2016
Department of Alternative Energy Development and Efficiency, Biomass potential in Thailand (2013) http://biomass.dede.go.th/biomass_web/index.html. Accessed 21 October 2016
Mukherjee A, Dumont M-J, Raghavan V (2015) Review: sustainable production of hydroxymethylfurfural and levulinic acid: challenges and opportunities. Biomass Bioenergy 72:143–183
Worasuwannarak N, Potisri P, Tanthapanichakoon W (2006) Upgrading of biomass by carbonization in hot compressed water. Songklanakarin J Sci Technol 28:1049–1057
Zhang L, Wang Q, Wang B et al (2015) Hydrothermal carbonization of corncob residues for hydrochar production. Energy Fuel 29:872–876
Gan J, Yuan W (2013) Operating condition optimization of corncob hydrothermal conversion for bio-oil production. Appl Energy 103:350–357
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. (2005) Determination of ash in biomass (NREL/TP-510-42622). The US National Renewable Energy Laboratory technical report
ASTM (2010) Standard test methods for proximate analysis of coal and coke by macro thermogravimetric analysis. Method D7582-10. ASTM International, Pennsylvania
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. (2008) Determination of structural carbohydrates and lignin in biomass (NREL/TP-510-42618). The US National Renewable Energy Laboratory technical report
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81:1051–1063
Xiao L-P, Shi Z-J, Xu F et al (2012) Hydrothermal carbonization of lignocellulosic biomass. Bioresour Technol 118:619–623
Nakason K et al (2017) Characteristics of hydrochar and liquid fraction from hydrothermal carbonization of cassava rhizome. J Energy Inst. doi:10.1016/j.joei.2017.01.002
Sermyagina E, Saari J, Kaikko J et al (2015) Hydrothermal carbonization of coniferous biomass: effect of process parameters on mass and energy yields. J Anal Appl Pyrolysis 113:551–556
Knežević D, Van Swaaij W, Kersten S (2010) Hydrothermal conversion of biomass. II. Conversion of wood, pyrolysis oil, and glucose in hot compressed water. Ind Eng Chem Res 49:104–112
Reza MT, Lynam JG, Uddin MH et al (2013) Hydrothermal carbonization: fate of inorganics. Biomass Bioenergy 49:86–94
Petrović J, Perišić N, Maksimović JD et al (2016) Hydrothermal conversion of grape pomace: detailed characterization of obtained hydrochar and liquid phase. J Anal Appl Pyrolysis 118:267–277
Kambo HS, Dutta A (2014) Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl Energy 135:182–191
Reza MT et al (2015) Hydrothermal carbonization (HTC) of wheat straw: influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour Technol 182:336–344
Reza MT et al (2014) Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour Technol 169:352–361
Beychok, M. (2012) Coal. http://www.eoearth.org/view/article/151276. Accessed 30 April 2016
Liu Z, Quek A, Kent Hoekman S et al (2012) Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour Technol 123:646–652
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Zhu Z, Liu Z, Zhang Y et al (2016) Recovery of reducing sugars and volatile fatty acids from cornstalk at different hydrothermal treatment severity. Bioresour Technol 199:220–227
Machmudah S, Wahyudiono W, Kanda H et al. (2015) Hot compressed water extraction of lignin by using a flow-through reactor. Engineering Journal; vol 19, No 4 (2015): regular issue. doi: 10.4186/ej.2015.19.4.25
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12
Lu X et al (2013) Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour Technol 138:180–190
Kim D, Lee K, Park KY (2016) Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J Ind Eng Chem 42:95–100
Liu Z et al (2013) Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 103:943–949
Pavlovic I, Knez Z, Skerget M (2013) Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: a review of fundamentals, mechanisms, and state of research. J Agric Food Chem 61:8003–8025
Antal MJ Jr, Mok WSL, Richards GN (1990) Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr Res 199:91–109
Hu L, Zhao G, Hao W et al (2012) Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv 2:11184–11206
Li K, Bai L, Amaniampong PN et al (2014) One-pot transformation of cellobiose to formic acid and levulinic acid over ionic-liquid-based polyoxometalate hybrids. ChemSusChem 7:2670–2677
Aida TM, Ikarashi A, Saito Y et al (2009) Dehydration of lactic acid to acrylic acid in high temperature water at high pressures. J Supercrit Fluids 50:257–264
Hisaya T, Pilasinee L, Tsuyoshi H et al (2014) Recovery of furfural produced by hydrothermal treatment with biomass charcoal. Int J Environ 4:11–17
Ju M, Zeng C, Wang C et al (2014) Preparation of ultrafine carbon spheres by controlled polymerization of furfuryl alcohol in microdroplets. Ind Eng Chem Res 53:3084–3090
U.S. Energy Information Administration (2016) Electric Power Monthly https://www.eia.gov/electricity/monthly/epm_table_grapher.cfm?t=epmt_5_6_a. Accessed 30 October 2016
Xin K et al (2016) Liquid–liquid equilibria for the extraction of furfural from aqueous solution using different solvents. Fluid Phase Equilib 425:393–401
Chan X et al (2016) Separation and purification of furfuryl alcohol monomer and oligomers using a two-phase extracting process. ACS Sustain Chem Eng 4(8):4084–4088
Sindermann EC et al (2016) Single stage and countercurrent extraction of 5-hydroxymethylfurfural from aqueous phase systems. Chem Eng J 283:251–259
Reyhanitash E et al (2016) Extraction of volatile fatty acids from fermented wastewater. Sep Purif Technol 161:61–68
Yano T et al (1989) Extraction of volatile fatty acids from spent medium with a supported liquid membrane. In: Fiechter A, Okada H, Tanner RD (eds) Bioproducts and bioprocesses: second conference to promote Japan/U.S. Joint Projects and Cooperation in Biotechnology, Lake Biwa, Japan, September 27–30, 1986. Springer, Berlin, pp 281–293
Cabezas JL et al (1988) Extraction of furfural from aqueous solutions using alcohols. J Chem Eng Data 33(4):435–437
Acknowledgements
The research project was supported by Mahidol University and Nanomaterial for Energy and Catalyst Laboratory, National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA). The study was supported for publication by the China Medical Board (CMB), Faculty of Public Health, Mahidol University, Bangkok, Thailand. Financial assistance for this research was also provided by the Center of Excellent on Environmental Health and Toxicology (EHT), Bangkok, Thailand.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 202 kb)
Rights and permissions
About this article
Cite this article
Nakason, K., Panyapinyopol, B., Kanokkantapong, V. et al. Characteristics of hydrochar and hydrothermal liquid products from hydrothermal carbonization of corncob. Biomass Conv. Bioref. 8, 199–210 (2018). https://doi.org/10.1007/s13399-017-0279-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-017-0279-1