Abstract
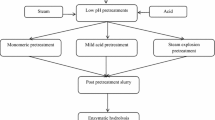
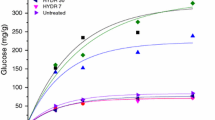
In lignocellulosic (LC) ethanol processes, to facilitate enzymatic hydrolysis of cellulose, a physical chemical pretreatment is vital. In this study, we explored a single as well as a two-step physical-chemical pretreatment involving steam and mixed acid on unwashed sugarcane bagasse at pilot-scale level in a continuous horizontal reactor. To serve as a large-scale model, pretreatments were carried out at high solid levels of 18–20 % w/w. For the pretreatment, partial replacement of corrosive sulfuric acid with a milder acid-like oxalic acid was explored to derive possible advantages and synergies accruing by using a mixture of mineral acid and organic acid. The results of this work showed that first-step pretreatment carried out by the mixing of sulfuric acid (1.5 % w/w) and oxalic acid (1.5 % w/w) at 150 °C followed by a second-step steam explosion pretreatment at 180 °C gave significant synergies and advantages over other variants of pretreatments investigated, such as lower inhibitor levels and lower reaction severity. On post-pretreated bagasse, this study conducted comparative enzymatic hydrolysis study using a simple lab enzyme and a robust commercial enzyme. It was found that the addition of Tween 80 to the lab enzyme improved its performance to match the performance of the commercial enzyme. Scanning electron microscopy (SEM) studies were further carried out to correlate the morphology of pretreated samples with efficiency of enzyme hydrolysis. Besides morphological study, Fourier transform infrared (FTIR) studies of pretreated samples showed higher syringyl/guaiacyl ratio for all pretreatments, indicating lower levels of pseudo-lignins, which is beneficial for improved enzyme hydrolysis.

Similar content being viewed by others
References
Kerr RA (2011) Peak oil production may already be here. Science 331:1510–1511
Sommerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329:790
Pandey A, Soccol CR, Nigam PE, SoccoL VT (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol 74(1):69–80
Dias MOS, Ensinas AV, Nebra SA, Maciel R, Rossell CEV, Maciel MRW (2009) Production of bioethanol and other bio-based materials from sugarcane bagasse: integration to conventional bioethanol production process. Chem Eng Res Des 87(9):1206–1216
Betancur GJV, Pereira JRN (2010) Sugarcane bagasse as feedstock for second generation ethanol production. Part I: diluted acid pretreatment optimization. Electronic Journal of Biotechnol 13(3):1–9
Rabelo SC, Carrere H, Maciel Filho R, Costa AC (2011) Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour Technol 102(17):7887–7895
Ferreria-Leitao V, Gottschalk LMF, Ferrara MA, Nepumuceno AL, Molinari HBC (2010) Biomass residues in Brazil: availability and potential uses. Waste Biomass Valor 1:65–76
Chandel AK, da Silva S, Carvalho W, Singh OV (2012) Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. J Chem Technol Biotechnol 87(1):11–20
Soccol CR, Vandenberghe LPDS, Medeiros ABP, Karp SG, Buckeridge M, Ramos LP, Pitarelo AP, Ferreira-Leitão V, Gottschalk LMF, Ferrara MA, Bon EPS, Moraes LMP, Araujo JA, Torres FAG (2010) Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresour Technol 101(13):4820–4825
Macrelli S, Mogensen J, Zacchi G (2012) Techno-economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol Biofuels 5:22
Rabelo SC, Amezquita-Fonseca NA, Andrade RR, Maciel-Filho R, Costa A (2011) Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 35(7):2600–2607
Teixieira LC, Linden JC, Herbert HA (1999) Optimizing peracetic acid treatment conditions for improved simultaneous saccharification and cofermentation of sugarcane bagasse to ethanol fuel. Renew Energy 16(1–4):1070–1073
Varma AJ (2009) Pretreatment of plant biomass carbohydrates for ethanol production: an overview. Trends in. Carbohydr Res 1(2):10–15
Chandel AK, Antunes FAF, Anjos V, Bell MJV, Rodrigues L, Polikarpov I, Azevedo de ER, Bernardinelli O, Rosa CA, Pagnocca FC, da Silva SS (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7:63
Wyman CE, Dale BE, Elander RT (2006) Coordinated development of leading biomass technologies. Bioresour Technol 96(18):1959–1966
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3372
Pedersen M, Viko-Nielsen A, Meyer AS (2010) Monosaccharide yields and lignin removal from wheat straw in response to catalyst type and pH during mild thermal pretreatment. Process Biochem 45(7):1181–1186
Chundawat SPS, Beckham GT, Himmel ME, Dale BE (2011) Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol Eng 2:121–145
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6(1)
Sousa LC, Chundawat SPS, Balan V, Dale BE (2009) Cradle-to-crave assessment of existing lignocellulose pretreatment technologies. Curr Opin Biotechnol 20(3):339–347
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Krishnan C, Sousa LC, Jin M, Chang L, Dale BE, Balan V (2010) Alkali-based AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnol Bioeng 107(3):441–450
Schell DJ, Farmer J, Newman M, McMillan JD (2003) Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor: investigation of yields, kinetics, and enzymatic digestibilities of solids. Appl Biochem Biotechnol 105–108:69–85
Rocha GJM, Nascimento VM, Silva VFN, Chandel AK (2014) Scale-up Pretreatment Studies on Sugarcane Bagasse and Straw for Second-Generation Ethanol Production. In: Fundamental aspects, recent developments, and future perspectives: biofuels in Brazil. Springer International, Switzerland, pp 225–254
Pal S, Joy S, Trimukhe KD, Kumbhar PS, Varma AJ, Padmanabhan S (2016) Effect of mixed acid catalysis on pretreatment and enzymatic digestibility of sugar cane bagasse. Energy and Fuels. doi:10.1021/acs.energyfuels.6b01011
Ye S, Jiayang C (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
Guo B, Zhang Y, Ha S-J, Jin Y-S, Morgenroth E (2012) Combined biomimetic and inorganic acids hydrolysis of hemicellulose in Miscanthus for bioethanol production. Bioresour Technol 110:278–287
Zhang T, Kumar R, Wyman CE (2013) Sugar yields from dilute oxalic acid pretreatment of maple wood compared to those with other dilute acids and hot water. Carbohydr Polym 92(1):334–344
Sluiter A, Hames B, Ruiz R, Scarlata, C, Sluiter J, Templeton D, Crocker D (2008) Determination of ash in energy laboratory, Laboratory Analytical Procedure (LAP), USA. (http://www.nrel.gov/biomass/pdfs/42622.pdf)
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass, National Renewable Energy Procedure (LAP). (http://www.nrel.gov/biomass/pdfs/42618.pdf)
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2005) Determination of extractives in biomass, National Renewable Energy Procedure (LAP) USA. (http://www.nrel.gov/biomass/pdfs/42619.pdf)
Mosier NS, Sarikaya A, Ladisch CM, Ladisch MR (2001) Characterization of dicarboxylic acids for cellulose hydrolysis. Biotechnol Prog 17(3):474–480
Lee JW, Houtman CJ, Kim HY, Choi IG, Jeffries TW (2011) Scale-up study of oxalic acid pretreatment of agricultural lignocellulosic biomass for the production of bioethanol. Bioresour Technol 102(16):7451–7456
Palmqvist E, Hagerdal BH (2000) Fermentation of lignocellulosic hydrolyzates. II. Inhibitors and mechanism of inhibition. Bioresour Technol 74:25–23
Tu M, Saddler JN (2010) Potential enzyme cost reduction with the addition of surfactant during the hydrolysis of pretreated softwood. Appl Biochem Biotechnol 161(1–8):274–287
Kaar WE, Holtzapple M (1998) Benefits from Tween during enzymatic hydrolysis of corn stover. Biotechnol Bioeng 59(4):419–427
Alkasrawi M, Eriksson T, Börjesson J, Wingren A, Galbe M, Tjerneld F, Zacchi G (2003) The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzym Microb Technol 33:71–78
Eriksson T, Borjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym Microb Technol 31:353–364
Park JW, Takahata Y, Kajiuchi T, Akehata T (1992) Effects of nonionic surfactant on enzymatic hydrolysis of used newspaper. Biotechnol Bioeng 39(1):117–120
Olesen SN, Bohlin C, Murphy L, Borch K, McFarland KC, Sweeny MD, Westh P (2011) Effects of non-ionic surfactants on the interaction between cellulases and tannic acid: a model system for cellulase-polyphenol interactions. Enzym Microb Technol 49:353–359
Singh R, Singh S, Trimukhe KD, Pandare KV, Bastawade KB, Gokhale DV, Varma AJ (2005) Lignin-carbohydrate complexes from sugarcane bagasse: preparation, purification, and characterization. Carbohydr Polym 62:57–66
Ghaffar SH, Fan M (2013) Structural analysis for lignin characteristics. Biomass Bioenergy 57:264–279
Studer MH, DeMartini JD, Davis MF, Sykes RW, Davison B, Keller M, Tuskan GA, Wyman CE (2011) Lignin content in natural Populus variants affects sugar release. Proc Natl Acad Sci U S A 108(15):6300–6305
Zhoa XB, Wang L, Liu DH (2008) Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: a continued work. J Chem Technol Biotechnol 83:950–956
Santos RB, Hart PW, Jameel H, Chang HM (2013) Wood based lignin reactions important to the biorefinery and pulp and paper industries. Bioresources 8(1):1456–1477
Li X, Ximenes E, Kim Y, Slininger M, Meilan R, Ladisch M, Chapple C (2010) Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol Biofuels 3
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23(6):1333–1339
Acknowledgments
The work presented here constitutes the doctoral research of SP under the supervision of AJV (academic institution) and of PK and SSP (Praj Matrix industrial research). All experimental samples were generated at M/s Praj Matrix. This latter part of the work was funded by M/s Praj Matrix. AJV and KDT thank the director of CSIR-NCL for providing the instrument facilities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Table S1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Pal, S., Joy, S., Kumbhar, P. et al. Pilot-scale pretreatments of sugarcane bagasse with steam explosion and mineral acid, organic acid, and mixed acids: synergies, enzymatic hydrolysis efficiencies, and structure-morphology correlations. Biomass Conv. Bioref. 7, 179–189 (2017). https://doi.org/10.1007/s13399-016-0220-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-016-0220-z