Abstract
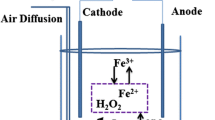
Sulfides are often found to be present in the effluents generated from some chemical and petrochemical industries as well as from tanneries. Beyond a certain permissible limit, sulfides are generally toxic to living bodies. In the present work, solution of sodium sulfide was first mixed with \(\hbox {Fe}^{+2}/\hbox {Fe}^{+3}\) salt solutions in different ratios which resulted in precipitation of sulfide from the solution. This was followed by aeration of the residual solution. The maximum sulfide removal efficiency of the \(\hbox {Fe}^{+2}/\hbox {Fe}^{+3}\) treatment was approximately 70 %. However, upon aeration, the residual sulfide was eventually removed. The precipitate formed was characterized as ferrous sulfide, applying scanning electron microscopy, energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy and X-ray power diffraction. The role of sulfide concentration, \(\hbox {Fe}^{+2}/\hbox {Fe}^{+3}\) ratio and temperature on the kinetics of sulfide removal from the aqueous solution was investigated. Effect of air flow rate during aeration of remaining sulfide was also investigated. The rate equation and activation energy for the precipitation reaction were calculated from the experimental results. The results of this study hold promise for effective treatment of sulfidic wastewater in chemical and allied industries.
Similar content being viewed by others
References
Salazar, J.R.; Mayers, R.A. (eds.): Handbook of Petroleum Processes, Part 9. McGraw-Hill, New York (1986)
Ksibi, M.: Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 119, 161–165 (2006)
Komnitsas, K.; Bazdanis, G.; Bartzas, G.: Efficiency of composite permeable reactive barriers for the removal of Cr(VI) from leachates. Desalin. Water Treat. 57, 8990–9000 (2016)
United states environmental protection agency, Centralized Waste Treatment Effluent Guidelines. https://www.epa.gov/eg/centralized-waste-treatment-effluent-guidelines
Zee, F.P.V.D.; Villaverde, S.; Garcia, P.A.; Polanco, F.F.: Sulfide removal by moderate oxygenation of anaerobic sludge. J. Bioresour. Technol. 98, 518–524 (2007)
Takenaka, N.; Furuya, S.; Sato, K.; Bandow, H.; Maeda, Y.; Furukawa, Y.: Rapid reaction of sulfide with hydrogen peroxide and formation of different final products by freezing compared to those in solution. J. Chem. Kinet. 35, 198–205 (2003)
Nielsen, A.H.; Jacobsen, T.H.; Vollertsen, J.: Effects of iron on chemical sulfide oxidation in waste water from sewer networks. J. Environ. Eng. 133, 655–658 (2007)
O’Brien, D.J.; Birkner, F.B.: Kinetics of oxygenation of reduced sulfur species in aqueous solution. Environ. Sci. Technol. 11, 1114–1120 (1977)
Bosch, P.L.F.V.D.; Picornell, M.F.; Janssen, A.J.H.: Effects of methanethiol on the biological oxidation of sulfide at natron alkaline conditions. J. Environ. Technol. 43, 453–459 (2009)
Chen, C.; Wang, A.; Ren, N.; Lee, D.J.; Lai, Y.: High-rate denitrifying sulfide removal process in expanded granular sludge bed reactor. J. Bioresour. Technol. 100, 2316–2319 (2009)
Munter, R.: Advanced oxidation processes–current status and prospects. J. Sci. Chem. 50, 59–80 (2001)
Baogang, Z.; Jing, Z.; Liu, Y.; Hao, C.; Tian, C.; Feng, C.; Lei, Z.; Wenli, H.; Zhang, Z.: Identification of removal principles and involved bacteria in microbial fuel cells for sulfide removal and electricity generation. Int. J. Hydrog. Energy 38, 14348–14355 (2013)
Takenaka, N.; Furuya, S.; Sato, K.; Bandow, H.; Maeda, Y.; Furukawa, Y.: Rapid reaction of sulfide with hydrogen peroxide and formation of different final products by freezing compared to those in solution. J. Chem. Kinet. 35, 198–205 (2003)
Zou, L.: Arsenic Removal from Industrial Waste Water by Photo Oxidation. Master thesis. Ottawa-carleton institute for civil and environmental engineering Carleton University Ottawa, Ontario Canada (2007)
Spiller, W.; Wohrle, D.; Ekloff, G.S.; Ford, W.T.; Schneider, G.; Stark, J.: Photo-oxidation of sodium sulfide by sulfonated phthalocyanines in oxygen saturated aqueous solution containing detergents or latexes. J. Photochem. Photobiol. A Chem. 95, 161–173 (1996)
Iliev, V.; Mihaylova, A.: Photooxidation of sodium sulfide and sodium thiosulfate under irradiation with visible light catalyzed by water soluble polynuclear phthalocyanine complexes. J. Photochem. Photobiol. A Chem. 149, 23–30 (2002)
Linkous, C.A.; Huang, C.; Flower, J.R.: UV photochemical oxidation of aqueous sodium sulfide to produce sulfur and hydrogen. J. Photochem. Photobiol. A Chem. 168, 153–160 (2004)
Nielsen, A.H.; Lens, P.; Vollertsen, J.; Jacobsen, H.: Sulfide-iron interactions in domestic wastewater from a gravity sewer. J. Water Res. 39, 2747–2755 (2005)
Shammas, N.K.; Yang, J.Y.; Yuan, P.C.; Hung, Y.T.: Photochemical treatment processes. Handbook of environmental engineering. 3, 229–270 (2007)
Ahmad, N.; Ahmad, F.; Khan, I.; Khan, A.D.: Studies on the oxidative removal of sodium thiosulfate from aqueous solution. Arab. J. Sci. Eng. 40, 289–293 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, F., Maitra, S. & Ahmad, N. Treatment of Sulfidic Wastewater Using Iron Salts. Arab J Sci Eng 42, 1455–1462 (2017). https://doi.org/10.1007/s13369-016-2315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-016-2315-z