Abstract
Hepatitis C virus-infected (HCV+) adults evidence increased rates of psychiatric and cognitive difficulties. This is the first study to use functional magnetic resonance imaging (fMRI) to examine brain activation in untreated HCV+ adults. To determine whether, relative to non-infected controls (CTLs), HCV+ adults exhibit differences in brain activation during a delay discounting task (DDT), a measure of one’s tendency to choose smaller immediate rewards over larger delayed rewards—one aspect of impulsivity. Twenty adults with HCV and 26 CTLs completed an fMRI protocol during the DDT. Mixed effects regression analyses of hard versus easy trials of the DDT showed that, compared with CTLs, the HCV+ group exhibited less activation in the left lateral occipital gyrus, precuneus, and superior frontal gyrus. There were also significant interactive effects for hard–easy contrasts in the bilateral medial frontal gyrus, left insula, left precuneus, left inferior parietal lobule, and right temporal occipital gyrus; the CTL group evidenced a positive relationship between impulsivity and activation, while the HCV+ group exhibited a negative relationship. Within the HCV+ group, those with high viral load chose immediate rewards more often than those with low viral load, regardless of choice difficulty; those with low viral load chose immediate rewards more often on hard choices relative to easy choices. Results show that HCV+ patients exhibit greater impulsive behavior when presented with difficult choices, and impulsivity is negatively related to activation in regions important for cognitive control. Thus, interventions that decrease impulsive choice may be warranted with some HCV+ patients.
Similar content being viewed by others
Introduction
The development of direct-acting antivirals (DAA) has markedly improved sustained viral response (SVR) rates in those receiving treatment for hepatitis C virus (HCV) (D’Ambrosio et al. 2017). However, universal access to treatments, even in wealthy countries, is unlikely in the foreseeable future due to high costs and other barriers (Chidi et al. 2016a, b; Gentile et al. 2016), and a minority of individuals (~ 1–9%) do not clear the virus even following treatment with DAA (D’Ambrosio, Degasperi et al. 2017). Thus, HCV continues to be a world-wide epidemic, affecting more than 185 million people (more than 2.8% of population) internationally (Mohd Hanafiah et al. 2013).
Our group and others have shown that untreated adults with HCV experience a range of psychiatric (Dwight et al. 2000; Poynard et al. 2002; Fireman et al. 2005; Golden et al. 2005; Rowan et al. 2005; Dan et al. 2006; Martin-Santos et al. 2008; Whitehead et al. 2008; Huckans et al. 2014) and cognitive symptoms (Forton et al. 2002; Martin et al. 2004; von Giesen et al. 2004; Weissenborn et al. 2004; Cherner et al. 2005; Letendre et al. 2005; McAndrews et al. 2005; Karaivazoglou et al. 2007; Huckans et al. 2009; Sakamoto et al. 2013). For example, we found that, compared with non-infected controls who were matched in terms of demographics and medical, psychiatric, and substance use history, untreated adults with HCV report significantly worse symptoms of depression, anxiety, fatigue, and pain (Huckans et al. 2014). Similarly, untreated adults with HCV demonstrated increased impairments on standard neuropsychological tests of memory, attention, and executive function, irrespective of drug use history, compared with controls whom were well matched on demographics and medical and psychiatric history (Huckans et al. 2009). Although the most common transmission route for HCV is injection drug use (IDU), these studies have generally demonstrated that HCV-associated neuropsychiatric impairments occur even after controlling for other factors such as medical, psychiatric, and substance use comorbidities.
Executive dysfunction, in particular, has been frequently noted in individuals with HCV on various neuropsychological tests, including those measuring verbal and non-verbal reasoning/problem-solving skills, abstraction, and mental flexibility (Weissenborn et al. 2004; Cherner et al. 2005; Letendre et al. 2005; Bieliauskas et al. 2006; Huckans et al. 2009). Impulsivity is regarded as another aspect of executive function, and several (Cordoba et al. 2003; Martin et al. 2004), but not all (Huckans et al. 2009), previous studies have found HCV-associated deficits on neuropsychological measures of response inhibition, one aspect of impulsivity. Other studies have shown that adults with HCV report increased behavioral symptoms of executive dysfunction, disinhibition, and impulsivity relative to non-infected controls (Posada et al. 2010; Fabregas et al. 2014; Dantas-Duarte et al. 2016). Delay discounting has been conceptualized as a behavioral model of impulsivity in which impulsive individuals are likely to choose smaller, less valuable immediate rewards over larger, more valuable delayed rewards (Ainslie 1975). Although delay discounting tasks (DDTs) vary, in general, the magnitude of an individual’s tendency towards delay discounting is commonly calculated as a function of the length of various delay periods as well as the value of various immediate versus delayed rewards (Reynolds 2006). Individuals with a variety of addictions have an increased tendency towards delay discounting (Hoffman et al. 2006; Reynolds 2006; Hoffman et al. 2008), and temporal discounting has been proposed as a behavioral marker of addiction (Bickel et al. 2014). In our own previous study, we found that untreated HCV-infected adults, including groups with and without a history of substance use disorders, also exhibited an increased preference for immediate rewards on the DDT, compared with well-matched (demographics, medical and psychiatric history) non-infected controls (Huckans et al. 2011). Likewise, one other study demonstrated that HCV infection (but not human immunodeficiency virus (HIV) infection or the interaction of HCV and HIV infection) was associated with more impulsive performance on the DDT among adults with current drug dependence (Martin et al. 2015).
The present study is a follow-up to our previous investigations, as described above. Specifically, our primary objective was to determine whether, relative to non-infected controls, individuals with HCV exhibit differences in brain activation during a DDT. Previous neuroimaging studies comparing untreated adults with HCV to non-infected controls have found a variety of HCV-associated changes using various methods (electroencephalogram (EEG) (Weissenborn et al. 2004), magnetic resonance spectroscopy (MRS) (Forton et al. 2001; Taylor et al. 2004; Weissenborn et al. 2004; McAndrews et al. 2005; Bladowska et al. 2013; Thames et al. 2015), multimodal MRI or diffusion tensor imaging (DTI) (Bladowska et al. 2013; Thames et al. 2015; Kharabian Masouleh et al. 2017; Kumar et al. 2017), perfusion-weighted MRI (Bladowska et al. 2013; 2014), positron emission tomography (PET) (Grover et al. 2012; Pflugrad et al. 2016)). Collectively, findings could be interpreted to suggest that HCV causes or is associated with central nervous system (CNS) inflammation, damage, and/or neurodegeneration. For example, HCV-associated increases in fractional anisotropy (FA) in the striatum were also associated with poorer language fluency and overall neuropsychological function in HCV-infected patients in one study (Thames et al. 2015), and HCV-associated EEG alterations were correlated with poorer cognition in HCV-infected patients in another study (Weissenborn et al. 2004). Alternatively, findings could suggest that markers may in part represent compensatory (protective) mechanisms of the CNS to HCV infection. For example, HCV-associated increases in microglial activation were associated with improved cognitive function in HCV-infected patients in one study (Pflugrad et al. 2016), and HCV-associated increases in functional connectivity were associated with better memory and attention performance in HCV-infected patients in another study (Kharabian Masouleh et al. 2017).
The present study adds to the neuroimaging literature on HCV by utilizing functional magnetic resonance imaging (fMRI) to examine brain activation during a DDT. One previous study used fMRI to show that, compared with an untreated HCV-infected group (n = 11), a group of HCV-infected adults being treated with interferon alpha therapy (n = 10) evidenced significantly increased activation of the anterior cingulate cortex (ACC) during a task of visuospatial attention (Capuron et al. 2005). While results could reflect a treatment effect, because of the cross-sectional design and lack of a non-infected control group, it is unclear whether differences may have existed prior to treatment due to other confounds or whether adults with HCV (treated or untreated) might differ from healthy controls without HCV. To our knowledge, the present study is the first fMRI study to examine brain activation in untreated HCV-infected adults compared with non-infected controls to assess HCV effects (rather than treatment effects). Impulsive choice has been proposed as a core feature of addiction as well as a risk factor for treatment failure (de Wit 2009; Newton et al. 2009; Ersche et al. 2010; Stevens et al. 2015). Bickel et al. (2012) has argued persuasively that neurobehavioral decision systems could be used as dimensional descriptors of addiction and impulsive choice. Models of impulsive decision making often posit an underactive cognitive control network, consisting of, e.g., prefrontal cortex and posterior parietal cortex and a more active salience attribution network comprised of, e.g., insula, striatum, and anterior cingulate cortex [e.g., McClure et al. 2004]. These networks have been identified in methamphetamine users (Hoffman et al. 2008), alcohol-dependent patients (Claus et al. 2011), and tobacco smokers (MacKillop et al. 2012). As neuroimaging findings in HCV-infected patients similarly suggest brain changes in prefrontal, posterior parietal, and striatal areas, we hypothesized that HCV-infected patients would evidence altered activation in these regions relative to non-infected controls on the DDT. We used a variation of the method of Hoffman et al. (Hoffman et al. 2008) to contrast hard choices (near the indifference curve) with easy choices (far from the indifference curve). This contrast controls for neural activation related to task performance (e.g., visual scanning, button pressing) but not to decision-making.
Despite limitations (e.g., studies are often correlational), there is growing recognition of the value of neuroimaging measures as potential biomarkers for neuropsychiatric disorders and treatments (Garrison and Potenza 2014). The present study examines brain activation during a DDT in untreated HCV patients without recent history (past year) of alcohol or drug dependence, because a better understanding of the neurobiological mechanisms of impulsive choice in this population may help to identify reasonable therapeutic targets. For example, if findings in this population are consistent with models of impulsive decision-making in other populations, then the development and evaluation of medications and behavioral treatments (e.g., cognitive rehabilitation interventions focused on decision-making, problem-solving, and planning skills) that improve cognitive control network activation may be warranted for HCV patients with executive impairments and/or high-risk behaviors. Although limited in size and scope, this is the first neuroimaging study to test the neurobiological model of impulsive decision-making in an HCV sample.
Methods
Subjects
Fifty subjects were recruited from the VA Portland Health Care System (VAPORHCS), the Oregon Health Clinic, and the community via study advertisements, mailings to patients who had previously participated in HCV research, announcements at hospital-based HCV education classes, or word of mouth. All subjects provided written informed consent approved by the VAPORHCS Institutional Review Board. The HCV group included 22 subjects with a positive diagnosis of HCV (verified by a detectable HCV viral load based on polymerase chain reaction (PCR) tests in their medical record or ordered through this study if recent records were unavailable) and probable infection of 5 years or more. The control (CTL) group included 28 subjects with no history of HCV infection (verified by a recent negative HCV antibody test in their medical record or ordered through this study if recent records were not available). Medical laboratory tests, including those used for HCV status verification, were collected as part of this study if the most recent tests in a participant’s medical record was from > 30 days prior to the study visit.
Exclusion criteria included previous history of interferon therapy or decompensated liver cirrhosis, as determined by the study hepatologist (AS) and relevant lab values (aspartate aminotransferase (AST), alanine transaminase (ALT), AST to Platelet Ratio Index (APRI)). Other exclusions included history of traumatic brain injury with loss of consciousness ≥ 30 min or suspected persistent cognitive impairment due to traumatic brain injury; color blindness or visual or auditory impairment that would prevent completion of cognitive tests; current pregnancy; cancer not currently in remission or history of chemotherapy; history of a major medical condition, or currently unstable medical condition, that is likely to be associated with significant cognitive, neurological, or immune dysfunction (e.g., brain tumor, stroke, multiple sclerosis, Parkinson’s disease, Huntington’s chorea, seizure disorder, dementia, or pervasive developmental disorders); acute medical illness likely to impact immune system; history of schizophrenia, schizoaffective disorder, or current Axis I psychiatric diagnosis, based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (American Psychiatric Association 2000), confirmed with the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998); alcohol or substance dependence if in remission for less than 365 days, based on DSM-IV criteria (American Psychiatric Association 2000), confirmed with the MINI (Sheehan et al. 1998); use of illicit drugs or alcohol intoxication within 24 h; current or regular use of antipsychotics, anticholinergics, stimulants, nitrate-containing medications, or other medications with acute cognitive effects such as sedation or intoxication. Subjects were also excluded for contraindications of MRI such as metal implants, pacemakers, and pregnancy.
Of note, this was not a treatment study, and this study was conducted prior to the availability of new DAAs. Thus, our participants were either (1) not planning to seek treatment (e.g., due to concerns for potential side effects; lack of social or other resources to support them during treatment; it was not a good time for them due to parental, work, or other responsibilities or priorities), (2) delaying treatment in the hopes that medications with better outcomes would become available, or (3) planning to start treatment soon but had not yet.
Procedures
All participants were reimbursed $150 to complete the following study procedures: clinical interview, medical record review, the Wechsler Test of Adult Reading (WTAR) (Holdnack 2001) to assess for baseline cognitive ability, the Generalized Anxiety Disorder 7-item Scale (GAD-7) (Spitzer et al. 2006) and the Beck Depression Inventory, Second Edition (BDI-II) (Beck et al. 1996) to assess for psychiatric symptom severity, a neuroimaging protocol, the delayed discounting (DDT) as described below, and a blood draw to allow for standard medical laboratory tests if unavailable by medical record.
Clinical interviews were conducted using a structured case report form, developed specifically for this study, including prompts to screen patients based on each inclusion criteria, gather relevant demographic data, evaluate for history of head injuries, and record a comprehensive list of current and previous medical conditions. Axis I psychiatric and substance use disorder diagnoses were assessed using the MINI (Sheehan et al. 1998), a well-validated structured clinical interview based on DSM-IV (American Psychiatric Association 2000) criteria. Study personnel additionally reviewed each participant’s medical record if available to collect medical laboratory results and to cross-validate the psychiatric, substance use, and medical history gathered in the clinical interview.
Delay discounting task—behavioral protocol
The DDT was initially administered as described in Hoffman et al. (Hoffman et al. 2008). Subjects were shown two options presented simultaneously on the left and right sides of a screen. One option offers a small reward available immediately, while the other option offers a larger reward (always $100) with a time delay between 1 and 365 days. From this data, an indifference curve is generated (Fig. 1) from a series of indifference points; an indifference point is the amount of money where the preference for the immediate or delayed choice is equal (Schwartz, Mitchell et al. 2010), as given in Eq. 1,
where SV is the subjective value of the delayed reward, k is a subject-specific measure of impulsivity, and d is the time (in days) to the larger delayed reward. After the initial administration, subjects performed additional runs in the lab where the maximum delay was lengthened or shortened until the subject chose nearly equal immediate and delayed options to better distribute data points. We used a softmax procedure (Miedl et al. 2014) implemented in MATLAB (Inc. 2013) to determine the value of k. Subject-specific impulsivity graphs and curves were generated using both the initial standard run and the final in-scanner behavioral data, and impulsivity (IMP) was defined as the fraction of total area above the indifference curve between 0 and 365 days (Schwartz et al. 2010).
Representation of a typical subject’s answers on the delay discounting task. As temporal delay increases, the number of immediate choices (green) increases, while the number of delayed choices (red) decreases. The indifference curve (black line) generated from subject responses reflects the points where subjects are equally likely to choose immediate or delayed options. The options near the indifference curve (pink) reflect hard choices while options distant from the indifference curve reflect easy choices
Delay discounting task—imaging protocol
The DDT was presented as an event-related fMRI task, with three 10.5-min runs. Each run included 116 choice pairs and 39 magnitude-estimation (ME) control trials, where both options were identical in either value or delay (e.g., $100 now OR $30 now). A jittered inter-trial interval varied between 0 and 4 s. The task was presented with Presentation software (Neurobehavioral Systems, Inc.).
MRI acquisition
Magnetic resonance imaging was acquired on a 3-T Siemens TIM Trio scanner. Functional T2*-weighted, echo-planar images (EPI) were acquired (TR = 2000 ms, TE = 50 ms, flip angle = 80°, FOV = 240 × 240 mm, 320 volumes and 24 slices of 4 mm thickness and 1 mm gap between slices). Three runs were collected in the scanner while performing the DDT. An anatomical magnetization-prepared rapid gradient-echo (MPRAGE) T1w scan was also acquired for coregistration to functional imaging (TR = 2300 ms, flip angle = 12°, FOV = 208 × 256 mm, 144 volumes, slice thickness of 1 mm, 176 slices and no gap between slices).
MRI processing
Image analysis was performed using the FMRIB Software Library (FSL; version 5.0; www.fmrib.ox.ac.uk/fsl). The image series from each participant was first realigned to compensate for small head movements (Jenkinson et al. 2002), and then high-pass temporal filtering (100 s) was applied. Data were spatially smoothed using a 5-mm FWHM Gaussian kernel, and skull-stripping was performed using the FSL Brain Extraction Tool. EPI images were first registered to the high-resolution MPRAGE structural image and then into standard Montreal Neurological Institute space. Statistical analyses were performed on data in native space using FMRIBs fMRI Expert Analysis Tool (FEAT), and the statistical maps were spatially normalized to standard space prior to higher-level analysis.
Image analysis
Four types of events or conditions were included in the general linear model (GLM): easy choice trials, hard choice trials, ME (control trial), and missed trials. Easy and hard choices were differentiated according to the methods of Hoffman et al. (Hoffman et al. 2008) whereby hard choices needed longer consideration (longer reaction times) and fall closer to the discounting curve than easy choices. The distance from the discounting curve, which was used to differentiate hard versus easy choices, was calculated from the distribution of reaction times for the in-scanner behavioral data and was set at $26 dollars. Hard choices were those within $26 of the indifference curve (the pink region in Fig. 1), while easy choices were those that were further away from the indifference curve.
Regressors were created by convolving a set of delta functions that represented the onset times of the events with a canonical (double-gamma) hemodynamic response function (HRF). The participant’s response time to decide determined the width of the HRF for each event. Additional regressors that represented the first temporal derivatives of the event-related regressors were included to capture variance associated with slight variations in the temporal lag of the hemodynamic response.
Whole-brain statistical analyses, using a fixed effects model, were conducted separately for each imaging run per participant and again to combine contrast images across the three runs. For between-participant analyses, the FMRIB Local Analysis of Mixed Effects module was used with lifetime substance dependence and current tobacco use status as covariates. Impulsivity was entered as a covariate in a separate model to examine group interactions with behavioral performance on task activation. Thresholds for statistical images were set at a voxel height of Z > 1.96 and a cluster-probability threshold of p < 0.05, corrected for whole-brain multiple comparisons using the Theory of Gaussian Random Fields.
Logistic regression
A mixed effects logistic regression was used to test for relationships between brain function and DDT behavior on a trial-by-trial basis. In the model, choices were modeled as immediate versus delayed choices for each trial while missed trials and ME trials were excluded. DDT behavior may be influenced by the difficulty of a decision (hard vs easy); therefore, choice difficulty was included in the model. The model also included parameter estimates from regions where there were significant group differences in activation in hard versus easy contrasts from the whole-brain voxel-wise group analyses. For analyses specific to the HCV group, subjects were grouped into either high or low viral load groups to test the effect of infection severity. One participant with HCV (antibody and PCR positive) was excluded from within group viral load comparisons due to a missing viral load value. DDT behavior was modeled as the dependent variable with viral load, group and choice difficulty as independent variables. This analysis tested the relationship between severities of infection on DDT behavior as a function of choice difficulty.
Results
Demographic characteristics
Final analyses included 20 HCV subjects and 26 CTL subjects after exclusions. Specifically, four subjects were excluded for unusable data in the scanner; two participants only chose immediate options, one randomly selected immediate and delays, and scan artifacts precluded the use of the fourth.
There were no statistically significant differences between the HCV group and CTL group in age, gender, race/ethnicity, education, psychiatric symptom severity (depression or anxiety), or estimated cognitive ability as measured by the WTAR (Holdnack 2001) (Table 1). Groups did not significantly differ in terms of rates of medical conditions or lifetime alcohol dependence. Lifetime drug dependence and current tobacco use were both significantly higher for the HCV group than for CTLs (Table 1); therefore, both were used as covariates in subsequent fMRI analyses. When broken down into substance categories, only rates of lifetime opiate dependence significantly differed between CTLs and the HCV group; rates of stimulant, marijuana, and other drug (hallucinogens, inhalants, and barbiturates) dependence did not significantly differ between groups. All subjects were in remission from substance dependence for at least 1 year, and the amount of time since dependence varied considerably in both groups (CTL = 8.34 ± 10.05 years, HCV = 9.39 ± 10.78 years).
The HCV group included subjects who had been infected with HCV for at least 5 years (mean = 26 years; standard deviation (SD) = 12.35).The following liver functioning data, expressed as mean ± standard deviation, were collected from recent medical records or ordered as part of the study visit: HCV ribonucleic acid (RNA) (log10 IU/ml) = 6.3 ± 0.9, AST = 61.3 ± 47.3, ALT = 85.7 ± 64.1, platelets (PLT) = 226.1 ± 97.6, and ammonia = 37.4 ± 12.9. Seventeen out of 20 subjects had HCV genotypes available in their medical record (9 with genotype 1, 5 with genotype 2, and 3 with genotype 3). The following risk factors were reported as non-mutually exclusive but possible transmission routes: IDU/shared drug paraphernalia (65%, n = 13), accidental exposure at work (5%, n = 1), blood transfusion (5%, n = 1), tattoos from non-commercial establishments or questionable sources (10%, n = 2), and other risk factors (15%, 1 intercourse with HCV+ spouse, 1 physical fight, 1 unknown).
Behavioral data—impulsivity, choice selection, and level of difficulty
On the initial DDT administration run (outside the scanner), the maximum delay period was standardized to 365 days across all subjects. On this initial run, groups did not significantly differ in terms of IMP (HCV = 0.430, CTL = 0.4660, t = 0.416, p = 0.679), indicating the groups did not initially differ in terms of impulsivity or, more specifically, their overall preference for delayed versus immediate rewards. A GLM analysis of this behavioral data revealed a significant main effect of difficulty on choice selection; both groups chose the immediate option more often when the decision was hard than when it was easy (t = 7.547, p < 0.001). The main effect of group was non-significant, but there was a significant difficulty by group interaction (t = − 2.345, p = 0.019) on choice selection. Specifically, the increased tendency (i.e., change in tendency) to pick immediate rewards on hard trials relative to easy trials was more pronounced in the HCV group compared with that in the CTL group (Fig. 2a).
By group, the percentage of times participants select immediate rewards over delayed rewards on hard versus easy trials on the delayed discounting task (DDT). Participants in both the hepatitis C virus (HCV) infected and non-infected control (CTL) groups have a significantly (p < 0.05) increased tendency to choose smaller immediate rewards over larger delayed rewards when choices are hard versus when they are easy. a Results from the initial administration of the DDT outside the scanner. b Results from the final administration of the DDT during functional magnetic resonance imaging (fMRI)
As described previously, the maximum delay period was then modified for each individual with additional runs outside the scanner until they chose an equal number of immediate versus delayed rewards; this maximum delay period was used for the final administration inside the scanner. IMP values from the initial administration (outside the scanner) and the final in-scanner administration were compared. There was a significant trend towards greater impulsivity between IMP from the initial administration to the in-scanner administration with all subjects combined (initial IMP = 0.450, scanner IMP = 0.613, t = −3.007 p = 0.003). A MANOVA with initial and scanner IMP as within subject repeated measures and group as a between-subject factor was significant (Wilk’s Lambda = 0.76, F (2,42) = 6.8, p = 0.003). The between-subject effect of group was non-significant (F (1,43) = 0.04, p = 0.6). There was a significant main effect of time (F (2,86) = 9.52, p < 0.001) but no time–group interaction (F (2,86) = 0.8, p = 0.4). We further examined the interaction using paired t tests. Both groups showed significantly greater impulsivity in the scanner, but the magnitude of the effect did not significantly differ between groups (CTL: initial IMP = 0.466, scanner IMP = 0.648, t = − 3.381, p = 0.002, HCV: initial IMP = 0.430, scanner IMP = 0.567, t = − 2.185, p = 0.042).
Within the scanner, overall, the groups were presented with a similar number of hard and easy choices; the CTL group had 47.5% hard choices and the HCV group had 46.4% hard choices. There were no significant group differences in the number of missed trials. As with the initial run, there were no significant differences between groups in terms of IMP on the final scanner administration (CTL = 0.648, HCV = 0.567, t = 1.189, p = 0.241). A GLM analysis of the behavioral data from the scanner revealed a significant main effect of difficulty on choice selection; both groups chose the immediate option more often when the decision was hard than when it was easy (t = 15.412, p < 0.001). There was also a significant main effect of group (t = − 3.600, p = 0.000) on choice selection; members of the HCV group made more immediate and therefore more impulsive choices than CTLs when level of difficulty was modeled (t = 7.495, p = 0.000). And, there was a significant difficulty by group interaction (t = 3.285, p = 0.001) on choice selection. Specifically, the increased tendency (i.e., change in tendency) to pick immediate rewards on hard trials relative to easy trials was more pronounced in the CTL group compared with that in the HCV group even though the HCV group was more likely to pick immediate rewards than CTLs in both conditions (Fig. 2b).
In-scanner IMP was used in follow-up analyses examining the effect of IMP on brain activation.
fMRI contrasts
We first ran a whole-brain contrast comparing activation on hard versus easy trials. For the hard–easy whole-brain contrast, the total sample (both groups combined) exhibited significant activation in the ACC, right medial frontal gyrus (MFG), right superior frontal gyrus (SFG), left insula, and left inferior frontal gyrus (IFG) (Fig. 3a).
Whole-brain contrast comparing activation on hard versus easy trials on the delayed discounting task (DDT). a Contrast of hard versus easy decisions for all subjects combined. Significant regions of activation for hard compared to easy choices include the anterior cingulate cortex, right middle frontal gyrus, right superior frontal gyrus, left insula, and left inferior frontal gyrus. b Group differences for the hard versus easy contrast. Compared to the hepatitis C virus (HCV)-positive group, the control (CTL) group exhibits greater differences in activation between hard and easy choices in the left lateral occipital gyrus, left precuneus, and left superior frontal gyrus (p < 0.05, whole-brain corrected)
A whole-brain group comparison was then run on the hard–easy contrast. When comparing the groups in the hard–easy contrast, the HCV group exhibited significantly reduced activation in the left lateral occipital gyrus, left precuneus, and the left SFG compared with the CTL group (Fig. 3b); there were no regions where the HCV group exhibited greater activation compared to CTLs.
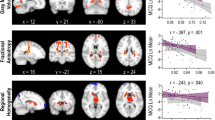
A whole-brain group by impulsivity (IMP) interaction was also run on the hard–easy contrast (whole-brain corrected at p < 0.05). In the hard–easy contrast, as IMP increased, activation decreased for the HCV group and increased for the CTL group (whole-brain mean activation in significant areas, Fig. 4). In other words, HCV subjects who acted more impulsively on the task had less differences in activation between hard and easy decisions than those who were less impulsive, while the opposite was true for CTLs; significant interactive effects were found in the bilateral MFG, left insula, left precuneus, left inferior parietal lobule, and the right temporal occipital gyrus.
Whole-brain group by impulsivity (IMP) interaction comparing activation on the hard versus easy trials on the delayed discounting task (DDT). a Clusters in which the group by IMP interaction was significant for the hard versus easy contrast on the DDT. b Within the clusters with a significant group by IMP interaction, the relationship between IMP and activation on the hard versus easy trials is positive for controls (CTLs) but negative for the hepatitis C virus (HCV)-infected group (p < 0.05, whole-brain corrected)
Exploratory analysis of the effect of viral load on behavior
The HCV subjects were then each classified into high viral load and low viral load groups. Most of our subjects would be considered high viral load by the standard of 800,000 IU/ml (5.9 log10 IU/ml); therefore, we split subjects on the mean of our sample (6.5 log10 IU/ml). Groups showed no significant differences on demographic variables (Table 1), except lifetime opiate dependence and depressive symptom severity. There were significant differences in IMP between the two viral groups (low = 0.67, high = 0.43, t = 2.307, p < 0.035). When further examining choice selection and the effect of difficulty, there was a significant main effect of viral load group on choice (t = − 3.813, p < 0.001) and a significant difficulty by viral load interaction (t = 4.298, p < 0.001). Controlling for opiate dependence and depressive symptom severity did not significantly impact results. Specifically, subjects in the high viral load group were more likely to choose the immediate option compared with those in the low viral load group (Fig. 5a), and the low viral load group had an increased tendency to choose the immediate option during hard trials compared to easy trials (Fig. 5b).
By viral load, the percentage of times participants infected with the hepatitis C virus (HCV) select smaller immediate rewards over larger delayed rewards on hard versus easy trials on the delayed discounting task (DDT). a Participants with a high viral load choose immediate rewards significantly (p < 0.001) more often than those with a low viral load. b Participants with a high viral load choose immediate rewards significantly (p < 0.001) more often during both hard trials and easy trials, whereas participants in the low viral load are more likely to choose immediate rewards during hard trials relative to easy trials
Discussion
Overall, our findings indicate that brain activation patterns of adults with HCV significantly differ from non-infected adults during a DDT. Specifically, compared with non-infected CTLs, our HCV group exhibited less activation in the left lateral occipital gyrus, left precuneus, and the left SFG in the hard–easy contrasts (Fig. 3b). Moreover, there were significant interactive effects for hard–easy contrasts in the bilateral MFG, left insula, left precuneus, left inferior parietal lobule, and the right temporal occipital gyrus such that more impulsive adults with HCV showed less differences in activation between hard and easy trials than less impulsive adults with HCV; in contrast, more impulsive CTLs showed more differences in activation between hard and easy trials than less impulsive CTLs (Fig. 4).
HCV-associated changes in brain activation are particularly noteworthy in our sample given our efforts to match groups in terms of important demographic, medical, psychiatric, and substance use characteristics. Our groups did not significantly differ in terms of age, gender, race/ethnicity, years of education, estimated cognitive ability, psychiatric symptom severity, or history of medical conditions. Since most individuals contract HCV through IDU, we excluded individuals with recent (< 1 year) drug dependence. Although the HCV group evidenced a significantly higher rate of remote drug dependence (in particular, opiate dependence) and current tobacco use, both groups included individuals with these addictions, and there were no significant differences across groups in terms of rates of remote alcohol dependence. Moreover, remote drug dependence and tobacco use were used as covariates in subsequent imaging analyses. Our efforts to match groups in terms of common HCV comorbidities likely resulted in a somewhat healthier HCV group than what might be seen in the general population. Indeed, to minimize differences between groups that might otherwise contribute to brain activation differences, we excluded all subjects with any history of psychiatric disorder other than a remote history of addiction. As we and others have shown, individuals with HCV exhibit higher rates of psychiatric symptoms such as depression, anxiety, fatigue, and pain, which has been correlated with inflammatory markers in some but not all studies (Dwight et al. 2000; Poynard et al. 2002; Fireman et al. 2005; Golden et al. 2005; Rowan et al. 2005; Dan et al. 2006; Martin-Santos et al. 2008; Whitehead et al. 2008; Huckans et al. 2014). Thus, our design purposely excluded individuals with typical HCV-associated psychiatric disorders, likely resulting in a healthier, less symptomatic HCV group overall.
Although exclusions and matching enhanced the likelihood that group differences in brain activation were due to HCV infection rather than other comorbidities, it may also have contributed to why the HCV and CTL groups did not display differences in IMP on the DDT in the present study. Indeed, in our previous study with a different design and sample, we found that individuals with HCV, regardless of a history of substance dependence, were significantly more likely to choose immediate over delayed rewards than non-infected controls (Huckans et al. 2011). While groups in this latter study were well matched in terms of demographics and medical history, we did not exclude for mild psychiatric disorders such as depression or anxiety, and we did not include controls with a history of any substance dependence. Thus, HCV-associated symptoms, including impulsivity, may have been pronounced in this latter study while being undetectable in the present less symptomatic sample. One other notable difference was that subjects in the previous study were all veterans from the VAPORHCS while participants in the present study were both veterans and non-veterans recruited from other health care centers and the community; it is unclear how this difference may have impacted the conflicting study findings on the DDT.
While we did not detect significant group differences in terms of IMP on the DDT in the present study, when modeling for choice difficulty, we did find significant differences in DDT performance between those with high versus low viral loads among those who were infected with HCV. When modeling for choice difficulty, we found that individuals in the high viral load group showed a greater propensity for immediate choices irrespective of difficulty, while the low viral load group showed a greater likelihood of choosing more immediate choices on difficult versus easy choices (similar to the pattern seen in CTLs). This finding suggests that severity of infection may contribute to impulsivity and decision-making; however, more research is needed to disentangle possible confounding variables such as drug use and exposure. Although the present study did not analyze inflammatory markers in relation to imaging findings and cannot confirm causative mechanisms, results are generally consistent with other studies demonstrating that viral load and peripheral inflammatory factors (e.g., cytokines, chemokines) are related to HCV-associated neuropsychiatric symptoms (Loftis et al. 2008; Hilsabeck et al. 2010; de Almeida et al. 2011; Huckans et al. 2014).
The present study is also generally consistent with previous neuroimaging studies demonstrating a range of HCV-associated CNS effects (Forton et al. 2001; Taylor et al. 2004; Weissenborn et al. 2004; McAndrews et al. 2005; Grover et al. 2012; Bladowska et al. 2013; 2014; Thames et al. 2015; Pflugrad et al. 2016; Kharabian Masouleh et al. 2017; Kumar et al. 2017) and with the literature on neuroimmune pathways leading to HCV-associated neuropsychiatric symptoms and CNS changes. Viruses can cross tight junctions due to high levels of viremia and inflammation, and HCV RNA has been detected in cerebrospinal fluid and brain parenchyma (Laskus et al. 2002; 2005; Letendre et al. 2007; Adinolfi et al. 2015). It has been proposed that HCV can cross the blood–brain barrier via infected circulating macrophages and monocytes (i.e., the “trojan horse” theory of HCV neuroinvasion) (Laskus et al. 2002; 2005; Letendre et al. 2007; Adinolfi et al. 2015) or through the involvement of exosomes (Shen et al. 2017). While brain endothelial cells express receptors that can facilitate HCV neuroinvasion and replication (Fletcher et al. 2012), there is little direct evidence showing that HCV replicates in the brain or that it is has direct neurotoxic effects on CNS cell function (Liu et al. 2014). Thus, other mechanisms for HCV-associated CNS changes and neuropsychiatric symptoms should also be considered—such as resultant neuroinflammation, altered metabolic pathways, or altered neurotransmitter systems (Adinolfi et al. 2015). Moreover, at least some HCV-associated neuroimaging findings could alternatively represent adaptive, compensatory, or protective mechanisms of the CNS to HCV (central or peripheral) infection.
Our findings may have clinical implications for providers working with patients with HCV. CTLs evidenced a positive relationship between impulsivity and activation (hard–easy contrast), while the HCV group evidenced a negative relationship. Does this mean CTLs attempt to exert more cognitive control in the face of hard choices, while HCV-infected adults reduce their effort or do not recognize that additional effort is needed when choices are difficult? Likewise, do individuals with lower viral loads attempt to exert more cognitive control in the face of hard choices, while those with higher viral loads reduce their effort or fail to increase their effort in the face of more difficult choices? Although these types of interpretations cannot be definitively confirmed without additional studies, they suggest that clinicians and researchers may want to consider interventions designed to enhance cognitive control and decrease impulsive decision-making in this population. For example, it may be useful to educate patients about the importance of slowing down to carefully evaluate the short-term and long-term costs and benefits of various choices when they are faced with challenging or complex life decisions, particularly when they are experiencing active HCV symptoms or cognitive difficulties. Our group is investigating the efficacy of compensatory cognitive training (CCT), a brief, manualized cognitive rehabilitation intervention for adults with mild cognitive impairments due to a variety of conditions such as combat-related traumatic brain injury, aging, depression, and addictions (Storzbach et al. 2017). CCT teaches participants skills to help them manage everyday problems in the areas of attention, memory, and executive functions. In the module on decision-making, participants practice using a worksheet to assist them with making real-life decisions; first, they define the decision and their possible choices, and then, they use a table to break down the potential short-term costs, short-term benefits, long-term costs, and long-term benefits of each choice, before ultimately deciding what to do. For quick decisions when worksheets are not feasible, participants practice asking themselves four questions in their head (i.e., How will this choice help me now? How will it help me later? How will it hurt me now? How will hurt me later?) Future studies could evaluate the efficacy of these types of brief decision-making training interventions with this population, and neuroimaging studies could evaluate whether such interventions impact brain activation patterns (e.g., increase activation of the cognitive control network) during decision-making tasks.
The present study has multiple strengths (efforts to match the control group, use of covariates) and is the first fMRI study to compare brain activation patterns in HCV-infected adults versus non-infected CTLs. A notable weakness, however, is that the design precludes us from determining whether group differences in brain activation patterns during the DDT are due to HCV-associated damage, compensatory mechanisms, or other factors. Additional limitations include a relatively small sample size (limits power, increases the risk of sampling error) and the need to replicate results, particularly with regard to the high versus low viral load analyses. It should additionally be noted that most of our HCV-infected participants had high viral loads by clinical standards, so it is unclear how results may have changed if we had more variability on that measure. Drug dependence diagnoses were significantly more prevalent in the HCV group, particularly for opioid use. Although we corrected for lifetime drug use diagnoses and current substance dependence was exclusionary, the extent to which differences in brain function can be attributed to past drug use is unclear. Moreover, we did not collect quantity and frequency data on recent or lifetime use of alcohol or drugs, and substance use can impact clinical outcomes even in the absence of diagnoses. Future studies with larger sample sizes would be required to accurately model this confound. Studies could also examine whether HCV-associated changes in brain activation during the DDT or other tasks translates to real-world decision-making (e.g., managing money), risk taking, and daily functioning.
Lastly, future studies could examine whether HCV-infected individuals demonstrate impulsiveness and brain activation changes during other gold standard performance measures of impulsiveness, such as the Iowa gambling task (IGT) (Bechara et al. 1994; Buelow and Suhr 2009; Wardle et al. 2010). The DDT is similar to the IGT in that both tasks ask participants to make decisions that impact potential monetary earnings; however, the DDT is not a gambling task and may not trigger emotional arousal to the extent the IGT does. Moreover, the IGT requires individuals to make choices based on ambiguous reward structures, whereas the DDT provides explicit information about the reward parameters for each choice. Thus, future studies could determine whether HCV status differentially impacts decision-making and brain activation during tasks based on factors such as emotional arousal, emotional distress, and reward or task ambiguity.
In summary, this study adds to current literature demonstrating that HCV is associated with changes in brain activation consistent with reduced cognitive control. Specifically, we found that compared with non-infected CTLs, adults with HCV exhibit differences in brain activation during a DDT and that HCV-infected adults with higher viral loads perform more impulsively on the DDT than those with lower viral loads when taking choice difficulty into consideration (i.e., individuals with lower viral loads are less likely to pick immediate rewards on easy choices relative to hard choices, while those with higher viral loads are more likely to pick immediate rewards regardless of choice difficulty).
References
Adinolfi LE, Nevola R, Lus G, Restivo L, Guerrera B, Romano C, Zampino R, Rinaldi L, Sellitto A, Giordano M, Marrone A (2015) Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol 21(8):2269–2280
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82(4):463–496
American Psychiatric Association (2000) Diagnostic and statistical manual for mental disorders. American Psychiatric Association, Washington, DC
Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50(1–3):7–15
Beck AT, Steer RA, Brown GK (1996) Manual for the BDI-II. TX, The Psychological Corporation, San Antonio
Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM (2012) Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther 134(3):287–297
Bickel WK, Moody L, Quisenberry A (2014) Computerized working-memory training as a candidate adjunctive treatment for addiction. Alcohol Res 36(1):123–126
Bieliauskas LA, Back-Madruga C, Lindsay KL, Snow KK, Kronfol Z, Lok AS, Padmanabhan L, Fontana RJ (2006) Clinical relevance of cognitive scores in hepatitis C patients with advanced fibrosis. J Clin Exp Neuropsychol 28(8):1346–1361
Bladowska J, Knysz B, Zimny A, Malyszczak K, Koltowska A, Szewczyk P, Gasiorowski J, Furdal M, Sasiadek MJ (2014) Value of perfusion-weighted MR imaging in the assessment of early cerebral alterations in neurologically asymptomatic HIV-1-positive and HCV-positive patients. PLoS One 9(7):e102214
Bladowska J, Zimny A, Knysz B, Malyszczak K, Koltowska A, Szewczyk P, Gasiorowski J, Furdal M, Sasiadek MJ (2013) Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: a pilot study. J Hepatol 59(4):651–657
Buelow MT, Suhr JA (2009) Construct validity of the Iowa gambling task. Neuropsychol Rev 19(1):102–114
Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH (2005) Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry 58(3):190–196
Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, Grant I, H. I. V. N. R. C. Group (2005) Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 64(8):1343–1347
Chidi AP, Bryce CL, Donohue JM, Fine MJ, Landsittel DP, Myaskovsky L, Rogal SS, Switzer GE, Tsung A, Smith KJ (2016a) Economic and public health impacts of policies restricting access to hepatitis C treatment for Medicaid patients. Value Health 19(4):326–334
Chidi AP, Rogal S, Bryce CL, Fine MJ, Good CB, Myaskovsky L, Rustgi VK, Tsung A, Smith KJ (2016b) Cost-effectiveness of new antiviral regimens for treatment-naive U.S. veterans with hepatitis C. Hepatology 63(2):428–436
Claus ED, Kiehl KA, Hutchison KE (2011) Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res 35(7):1209–1219
Cordoba J, Flavia M, Jacas C, Sauleda S, Esteban JI, Vargas V, Esteban R, Guardia J (2003) Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol 39(2):231–238
D’Ambrosio R, Degasperi E, Colombo M, Aghemo A (2017) Direct-acting antivirals: the endgame for hepatitis C? Curr Opin Virol 24:31–37
Dan AA, Martin LM, Crone C, Ong JP, Farmer DW, Wise T, Robbins SC, Younossi ZM (2006) Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol 44(3):491–498
Dantas-Duarte A, Morais-de-Jesus M, Nunes AP, Miranda-Pettersen K, Araujo-de-Freitas L, Netto LR, Santos CT, Codes L, Quarantini LC (2016) Risk-taking behavior and impulsivity among HCV-infected patients. Psychiatry Res 243:75–80
de Almeida CM, de Lima TA, Castro DB, Torres KL, da Silva Braga W, Peruhype-Magalhaes V, Teixeira-Carvalho A, Martins-Filho OA, Malheiro A (2011) Immunological/virological peripheral blood biomarkers and distinct patterns of sleeping quality in chronic hepatitis C patients. Scand J Immunol 73(5):486–495
de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14(1):22–31
Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ (2000) Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res 49(5):311–317
Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW (2010) Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry 68(8):770–773
Fabregas BC, Abreu MN, Dos Santos AK, Moura AS, Carmo RA, Teixeira AL (2014) Impulsiveness in chronic hepatitis C patients. Gen Hosp Psychiatry 36(3):261–265
Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P (2005) Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis 40(Suppl 5):S286–S291
Fletcher NF, Wilson GK, Murray J, Hu K, Lewis A, Reynolds GM, Stamataki Z, Meredith LW, Rowe IA, Luo G, Lopez-Ramirez MA, Baumert TF, Weksler B, Couraud PO, Kim KS, Romero IA, Jopling C, Morgello S, Balfe P, McKeating JA (2012) Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology 142(3):634–643 e636
Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD (2001) Evidence for a cerebral effect of the hepatitis C virus. Lancet 358(9275):38–39
Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, Wesnes KA, Taylor-Robinson SD (2002) Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology 35(2):433–439
Garrison KA, Potenza MN (2014) Neuroimaging and biomarkers in addiction treatment. Curr Psychiatry Rep 16(12):513
Gentile I, Maraolo AE, Niola M, Graziano V, Borgia G, Paternoster M (2016) Limiting the access to direct-acting antivirals against HCV: an ethical dilemma. Expert Rev Gastroenterol Hepatol 10(11):1227–1234
Golden J, O’Dwyer AM, Conroy RM (2005) Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry 27(6):431–438
Grover VP, Pavese N, Koh SB, Wylezinska M, Saxby BK, Gerhard A, Forton DM, Brooks DJ, Thomas HC, Taylor-Robinson SD (2012) Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J Viral Hepat 19(2):e89–e96
Hilsabeck RC, Anstead GM, Webb AL, Hoyumpa A, Ingmundson P, Holliday S, Zhang Q, Casas AM, Jovel M, Stern SL (2010) Cognitive efficiency is associated with endogenous cytokine levels in patients with chronic hepatitis C. J Neuroimmunol 221(1–2):53–61
Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188(2):162–170
Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH (2008) Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology 201(2):183–193
Holdnack H (2001) Wechsler test of adult reading: WTAR. TX, The Psychological Corporation, San Antonio
Huckans M, Fuller BE, Olavarria H, Sasaki AW, Chang M, Flora KD, Kolessar M, Kriz D, Anderson JR, Vandenbark AA, Loftis JM (2014) Multi-analyte profile analysis of plasma immune proteins: altered expression of peripheral immune factors is associated with neuropsychiatric symptom severity in adults with and without chronic hepatitis C virus infection. Brain Behav 4(2):123–142
Huckans M, Seelye A, Parcel T, Mull L, Woodhouse J, Bjornson D, Fuller BE, Loftis JM, Morasco BJ, Sasaki AW, Storzbach D, Hauser P (2009) The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. J Int Neuropsychol Soc 15(1):69–82
Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Mitchell A, Lahna D, Johnson A, Loftis J, Woods SP, Mitchell SH, Hoffman W (2011) Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. J Clin Exp Neuropsychol 33(2):176–186
Inc., T. M. (2013). MATLAB release 2013a. Natick, Massachusettes, USA, The MathWorks, Inc.
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Karaivazoglou K, Assimakopoulos K, Thomopoulos K, Theocharis G, Messinis L, Sakellaropoulos G, Labropoulou-Karatza C (2007) Neuropsychological function in Greek patients with chronic hepatitis C. Liver Int 27(6):798–805
Kharabian Masouleh S, Herzig S, Klose L, Roggenhofer E, Tenckhoff H, Kaiser T, Thone-Otto A, Wiese M, Berg T, Schroeter ML, Margulies DS, Villringer A (2017) Functional connectivity alterations in patients with chronic hepatitis C virus infection: a multimodal MRI study. J Viral Hepat 24(3):216–225
Kumar A, Deep A, Gupta RK, Atam V, Mohindra S (2017) Brain microstructural correlates of cognitive dysfunction in clinically and biochemically normal hepatitis C virus infection. J Clin Exp Hepatol 7(3):198–204
Laskus T, Radkowski M, Adair DM, Wilkinson J, Scheck AC, Rakela J (2005) Emerging evidence of hepatitis C virus neuroinvasion. AIDS 19(Suppl 3):S140–S144
Laskus T, Radkowski M, Bednarska A, Wilkinson J, Adair D, Nowicki M, Nikolopoulou GB, Vargas H, Rakela J (2002) Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. J Virol 76(19):10064–10068
Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E, H. I. V. N. R. C. Group (2007) Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis 196(3):361–370
Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Heaton RK, McCutchan JA, Grant I, H. Group (2005) The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS 19(Suppl 3):S72–S78
Liu Z, Zhao F, He JJ (2014) Hepatitis C virus (HCV) interaction with astrocytes: nonproductive infection and induction of IL-18. 20(3):278–293
Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P (2008) Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. 430(3):264–268
MacKillop J, Amlung MT, Wier LM, David SP, Ray LA, Bickel WK, Sweet LH (2012) The neuroeconomics of nicotine dependence: a preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. 202(1):20–29
Martin-Santos R, Diez-Quevedo C, Castellvi P, Navines R, Miquel M, Masnou H, Soler A, Ardevol M, Garcia F, Galeras JA, Planas R, Sola R (2008) De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. 27(3):257–265
Martin E, Gonzalez R, Vassileva J, Bechara A (2015) Delay discounting is greater among drug users seropositive for hepatitis C but not HIV. 29(6):926–932
Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic S, Nunnally G, Sworowski L (2004) Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. 10(2):298–300
McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ (2005) Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. 41(4):801–808
McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004) Separate neural systems value immediate and delayed monetary rewards. 306(5695):503–507
Miedl SF, Fehr T, Herrmann M, Meyer G (2014) Risk assessment and reward processing in problem gambling investigated by event-related potentials and fMRI-constrained source analysis. 14:229
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. 57(4):1333–1342
Newton TF, De La Garza R 2nd, Kalechstein AD, Tziortzis D, Jacobsen CA (2009) Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse. 18(4):294–300
Pflugrad H, Meyer GJ, Dirks M, Raab P, Tryc AB, Goldbecker A, Worthmann H, Wilke F, Boellaard R, Yaqub M, Berding G, Weissenborn K (2016) Cerebral microglia activation in hepatitis C virus infection correlates to cognitive dysfunction. 23(5):348–357
Posada C, Moore DJ, Woods SP, Vigil O, Ake C, Perry W, Hassanein TI, Letendre SL, Grant I, H. I. V. N. R. C. Group (2010) Implications of hepatitis C virus infection for behavioral symptoms and activities of daily living. 32(6):637–644
Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, Ghillani P, Charlotte F, Piette JC, Moussalli J, Multivirc g (2002) Fatigue in patients with chronic hepatitis C. 9(4):295–303
Reynolds B (2006) A review of delay-discounting research with humans: relations to drug use and gambling. 17(8):651–667
Rowan PJ, Al-Jurdi R, Tavakoli-Tabasi S, Kunik ME, Satrom SL, El-Serag HB (2005) Physical and psychosocial contributors to quality of life in veterans with hepatitis C not on antiviral therapy. 39(8):731–736
Sakamoto M, Woods SP, Kolessar M, Kriz D, Anderson JR, Olavarria H, Sasaki AW, Chang M, Flora KD, Loftis JM, Huckans M (2013) Protective effects of higher cognitive reserve for neuropsychological and daily functioning among individuals infected with hepatitis C. 19(5):442–451
Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF (2010) Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. 50(4):1392–1401
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. 59(Suppl 20):22–33 quiz 34–57
Shen J, Huang CK, Yu H, Shen B, Zhang Y, Liang Y, Li Z, Feng X, Zhao J, Duan L, Cai X (2017) The role of exosomes in hepatitis, liver cirrhosis and hepatocellular carcinoma. 21(5):986–992
Spitzer RL, Kroenke K, Williams JB, Lowe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. 166(10):1092–1097
Stevens L, Goudriaan AE, Verdejo-Garcia A, Dom G, Roeyers H, Vanderplasschen W (2015) Impulsive choice predicts short-term relapse in substance-dependent individuals attending an in-patient detoxification programme. 45(10):2083–2093
Storzbach D, Twamley EW, Roost MS, Golshan S, Williams RM, O'Neil M, Jak AJ, Turner AP, Kowalski HM, Pagulayan KF, Huckans M (2017) Compensatory cognitive training for operation enduring freedom/operation Iraqi freedom/operation new Dawn veterans with mild traumatic brain injury. 32(1):16–24
Taylor MJ, Letendre SL, Schweinsburg BC, Alhassoon OM, Brown GG, Gongvatana A, Grant I, Hnrc (2004) Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. 10(1):110–113
Thames AD, Castellon SA, Singer EJ, Nagarajan R, Sarma MK, Smith J, Thaler NS, Truong JH, Schonfeld D, Thomas MA, Hinkin CH (2015) Neuroimaging abnormalities, neurocognitive function, and fatigue in patients with hepatitis C. 2(1):e59
von Giesen HJ, Heintges T, Abbasi-Boroudjeni N, Kucukkoylu S, Koller H, Haslinger BA, Oette M, Arendt G (2004) Psychomotor slowing in hepatitis C and HIV infection. 35(2):131–137
Wardle MC, Gonzalez R, Bechara A, Martin-Thormeyer EM (2010) Iowa gambling task performance and emotional distress interact to predict risky sexual behavior in individuals with dual substance and HIV diagnoses. 32(10):1110–1121
Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, Ahl B, Manns MP, Boker KW (2004) Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. 41(5):845–851
Whitehead AJ, Dobscha SK, Morasco BJ, Ruimy S, Bussell C, Hauser P (2008) Pain, substance use disorders and opioid analgesic prescription patterns in veterans with hepatitis C. 36(1):39–45
Acknowledgments
The authors would like to thank Jonathan Woodhouse and Adriana Seelye for their help with study start-up and data collection; Betsy Zucker, Patricia Taylor-Young, and the other providers of the VAPORHCS Hepatology Clinic for their continued collaboration and support of this research program; Janice Voukidis and the other providers of the Oregon Clinic’s Gastroenterology Clinic for help with recruitment and data collection; and Diane Howieson, Daniel Storzbach, Alexander Stevens, and Peter Hauser for their mentorship and essential input into initial study design.
Funding
This material was supported in part by the U.S. Department of Veterans Affairs VA Career Development Award Program (MH, JL) and IK2CX001790 (MK), Clinical Sciences Research and Development Merit Review Program CX001558-01A1 (WH), Biomedical Laboratory Research and Development Merit Review Program I01 BX002061 (JL), DOJ 2010-DD-BX0517 (WH), NIDA P50DA18165 (MH, WH, JL), NIDA T32 DA007262 (MK), NIAAA T32 AA007468 (MK), Collins Medical Trust (MK) and the Medical Research Foundation (MK). HM, MK, LD, DK, HL, and RA were employed as Research Assistants, MK, JL, and AV as Research Scientists, MC and AS as Staff Hepatologists, WF as a Staff Psychiatrist, and MH as a Staff Psychologist and Neuropsychologist at the Veterans Affairs Portland Health Care System, Portland, Oregon. Contents do not represent the view of the U.S. Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
McCready, H., Kohno, M., Kolessar, M. et al. Functional MRI and delay discounting in patients infected with hepatitis C. J. Neurovirol. 24, 738–751 (2018). https://doi.org/10.1007/s13365-018-0670-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-018-0670-0