Abstract
Tissue imaging using matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) is a well-established technique that, in recent years, has seen wider adoption and novel application. Applications such imaging mass spectrometry (IMS) and biotyping are beginning to gain greater exposure and use; however, with limitations in optimization methods, producing the best result often relies on the ability to customize the physical characteristics of the instrumentation, a task that is challenging for most mass spectrometry laboratories. With this in mind, we have described the effect of making simple adjustments to the laser optics at the final collimating lens area, to adjust the laser beam size and shape in order to allow greater customization of the instrument for improving techniques such as IMS. We have therefore been able to demonstrate that improvements can be made without requiring the help of an electrical engineer or external funding in a way that only costs a small amount of time.

ᅟ
Similar content being viewed by others
Introduction
Matrix assisted laser desorption/ionization mass spectrometry is a technique that is see a resurgence through application in a number of novel fields such as imaging mass spectrometry [1]. Initially hailed as the next revolution in histological imaging [2], it has become apparent in recent years that despite its wide applicability and ability to analyze a range of biomolecules from proteins to drugs [3,4,5,6,7,8], there are still a number of key impediments that have not yet been resolved [9]. Other techniques such as the analysis of microorganisms (known as biotyping [10]) is also expanding; however, the same problems exist.
Spatial resolution, which has only recently been defined [11], and limits of detection are two such concerns that appear to rely on the development of new instrumentation rather than improvements in analyses or sample preparation techniques [12]. While some instruments are capable of adjusting their laser to increase spatial resolution [13], the effect of this is a reduction in the laser diameter and therefore the number of molecular species that are ionised. This adjustment is also heavily controlled and characterized by a somewhat arbitrary scale of “ultra” to “very small”. While this may appear to be the ideal compromise, sacrificing diversity of molecular species for the sake of increased spatial information, the approach is limited to a single instrument manufacturer and is not applicable or implementable elsewhere [13]. Other attempts have been made to try and reduce laser spot size utilizing custom apparatuses and purposely built optical equipment [14,15,16,17]; however, this remains challenging, with the help of engineers and photonics experts. We therefore propose a simple explanation of the effect of manually changing the external focusing optics at the final collimating lens and the potential benefit this can have in improving spatial resolution without needing to physically reduce the size of the laser beam.
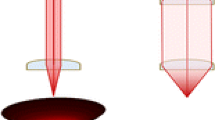
Modifying the internal construction of any complex analytical instrument is not a task for the average scientist; however, small adjustments can be made to the external focusing lenses that serve to modify the shape of the laser beam from a circle to an oblong profile as well as adjusting the focus of the beam for a more complete level of ionization. The advantage of this is that the area of sample that remains un-ablated between each ablation position (Figure 1) is reduced. This ensures that, at the same effective spatial resolution, more ions are generated as more of the previously inaccessible sample is being ionized. We therefore describe the effect of adjusting the final collimating lens (responsible for lineating and refocusing the laser beam) in the Sciex 5800 MALDI TOF/TOF system. The authors acknowledge that this process will be instrument-specific and therefore we provide this as a characterization of the effect as opposed to the actual process of adjusting the lenses.
Method
Preparation of Slides
To characterize the shape and size of the laser in reference to its ability to ionize a given sample, a method was devised whereby conductive indium tin oxide (ITO) microscope slides (laser biolabs, France) were coated with a homogenous layer of sinapinic acid matrix via sublimation, utilizing a previously described apparatus [3]. In short, the slide was first weighed on a 5-point analytical balance and the mass recorded. It was then affixed to the cooling finger of the sublimation apparatus. Three hundred mg of sinapinic acid matrix was placed in a petri dish at the bottom the chamber. The sublimator was then assembled and evacuated to ~25 mTorr for 5 min. Ice was placed into the cooling finger and sublimation was performed at 180 °C for 45 min, which should achieve an ideal coating of 2.2mg/cm2. Once completed, the chamber was disassembled and the slide was then re-weighed to ensure the correct amount of matrix had been deposited.
Instrumentation
A rectangle of matrix 500 μm × 100 μm (roughly 2 lines at 100 μm raster width) was then ablated in a Sciex 5800 MALDI TOF/TOF (Sciex MA USA) mass spectrometer in linear imaging mode with the following settings: 400 laser shots at 5100 laser power at a spatial resolution of 100 μm. The 5800 is equipped with solid state 355 nm Nd YAG laser with a repetition rate of 1000 Hz. Laser profile is Gaussian when used at default settings and prior to any adjustment of the internal focusing optics. The final collimating lens comes pre-installed in the instrument and is adjustable by loosening a small screw and manually adjusting the distance between the outlet of the laser and the lens (Figure 1).
Microscopy
Following laser ablation, the slide was imaged in a Nikon Instruments Eclipse Ti-E Inverted Microscope, at 40 × magnification with images captured using a Photometrics Cascade:1 K EMCCD Imaging Camera (Coherent Scientific SA, Australia). The discreet ablated spots were then measured and images were captured.
Results and Discussion
Once the images of the ablated slides were captured and ablation spot diameters measured, an immediate trend was recognized. Focusing was performed by using the in-built arbitrary unit scale on the bracket that contained the final collimating lens. The bracket is able to be slid up and down, lengthening and shortening the distance between the laser output and the sample in increments on a range of –9 to +25. By adjusting the laser in increments of 5 units, it was easy to see the effect of adjusting this distance.
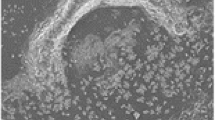
By default the laser was set to +5 and this produced the most “round” and even laser profile with a diameter of 100 μm (Figure 2); however, it also produced a significant Gaussian “corona” of partial ionization. We have described the phenomena previously [11] and find that the results we have collected in this investigation are consistent with our previous findings; i.e., despite the diameter of the laser being 100 μm, the area of ablation is closer to ~85 μm
Ablation of matrix at +5 setting. It can be clearly seen that the spots are round and 100 μm in diameter. There is also a significant area of partial ablation, which is consistent with the known Gaussian distribution of the laser spot intensity [13]
The laser was then sampled at both extremes of the range of movement, producing starkly different results, which could each be equally useful depending on the desired result.
At +25, the greatest distance between the laser output and the final lens, the shape of the beam changes significantly; showing a distinctly oval shape with a maximum width of 76 μm that leaves less space between the rows of ablation (Figure 3.). The spots still show a large corona of partial ionization; however, by being closer together, less matrix is left behind.
When the laser was adjusted to the opposing extreme of –9, the shortest distance between the laser and final lens, the laser profile was far more uniform and while still being 100 μm, had much sharper edges and less of a corona effect (Figure 4).This indicated that the laser was more focused and was able to ionize a greater proportion of its 100 μm diameter.
The spots also appear to be more uniformly ablated, which, as we have characterized previously [11], has potential implications for the integrity of the data analyzed from subsequent ablation locations.
From the above results, it is clear that by making minor adjustments to the optics of the instrument, significantly different effects can be achieved. The advantage of this is that the laser can be manually tuned to suit the intended purpose. In the case of a –9 adjustment, this could be applied to a sample where a high abundance known target needs to be imaged looking for presence or absence with maximum spatial resolution. In this case total ionization is not needed to maximize signal intensity as there is already sufficient ionization to generate high quality signal, even with the diminished area of effective ionization.
Conversely to the above, a +25 signal would be most beneficial when the most accurate relative quantitative result is needed for a wide spread of unknown molecules. In this case, total ionization of the sample is important as it localizes the ions within a particular spot (in this case it is limited to the 100 μm laser diameter) to its respective pixel, without leaving anything behind.
Conclusion
Our primary motivation for conducting this work was to continue investigating ways of improving several aspects of MALDI-MS. Although it is now a reasonably well-established technique, understanding the mechanisms behind laser ablation patterns [11] and other physiochemical phenomena are relatively new.
As we initially stated, the acquisition or modification of new instrumentation is an expensive undertaking that is beyond the reach or ability of many smaller research institutions. With this in mind, we hope that we have demonstrated that it is possible to tweak complex instrumentation in minor ways that can have significant impacts, allowing a higher level of customization and ultimately extending the range of application and improving existing data without the need for expensive or potentially destructive physical modifications to the instrument.
References
Caprioli, R.M., Farmer, T.B., Gile, J.: Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal.Chem. 69, 4751–4760 (1997)
Schwartz, S.A., Reyzer, M.L., Caprioli, R.M.: Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J. Mass Spectrom. 38, 699–708 (2003)
O'Rourke, M.B., Raymond, B.B.A., Djordjevic, S.P., Padula, M.P.: A versatile cost-effective method for the analysis of fresh frozen tissue sections via matrix-assisted laser desorption/ionization imaging mass spectrometry. Rapid Commun. Mass Spectrom. 29, 637–644 (2015)
O'Rourke, M.B., Djordjevic, S.P., Padula, M.P.: A non-instrument-based method for the analysis of formalin-fixed paraffin-embedded human spinal cord via matrix-assisted laser desorption/ionization imaging mass spectrometry. Rapid Commun. Mass Spectrom. 29, 1836–1840 (2015)
Powers, T.W., Neely, B.A., Shao, Y., Tang, H., Troyer, D.A., Mehta, A.S., Haab, B.B., Drake, R.R.: MALDI imaging mass spectrometry profiling of N-glycans in formalin fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One. 9, e106255 (2014)
Jackson, S.N., Barbacci, D., Egan, T., Lewis,E.K., Schultz, J.A., Woods, A.S.: MALDI-ion mobility mass spectrometry of lipids in negative ion mode. Anal. Methods: advancing methods and applications 6, 5001–5007 (2014)
Weaver, E.M., Hummon, A.B.: Imaging mass spectrometry: from tissue sections to cell cultures. Adv. Drug Deliv. Rev. 65, 1039–1055 (2013)
Watrous, J.D., Dorrestein, P.C.: Imaging mass spectrometry in microbiology. Nat. Rev. Microbiol. 9, 683–694 (2011)
O’Rourke, M.B., Djordjevic, S.P., Padula, M.P.: The quest for improved reproducibility in MALDI mass spectrometry. Mass Spectrom. Rev. (2016). https://doi.org/10.1002/mas.21515
Seng, P., Drancourt, M., Gouriet, F., La Scola, B., Fournier, P.E., Rolain, J.M., Raoult, D.: Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 49, 543–551 (2009)
O'Rourke, M.B., Raymond, B.B., Padula, M.P.: The Characterization of Laser Ablation Patterns and a New Definition of Resolution in Matrix Assisted Laser Desorption Ionization Imaging Mass Spectrometry (MALDI-IMS). J Am Soc Mass Spectrom. 28, 895–900 (2017)
Zavalin, A., Yang, J., Caprioli, R.: Laser beam filtration for high spatial resolution MALDI imaging mass spectrometry. J Am Soc Mass Spectrom. 24, 1153–1156 (2013)
Holle, A., Haase, A., Kayser, M., Hohndorf, J.: Optimizing UV laser focus profiles for improved MALDI performance. J Mass Spectrom. 41, 705–716 (2006)
Feenstra, A.D., Duenas, M.E., Lee, Y.J.: Five Micron High Resolution MALDI Mass Spectrometry Imaging with Simple, Interchangeable. Multi-Resolution Optical System. J Am Soc Mass Spectrom. 28, 434–442 (2017)
OuYang, C., Chen, B., Li, L.: High Throughput In Situ DDA Analysis of Neuropeptides by Coupling Novel Multiplex Mass Spectrometric Imaging (MSI) with Gas-Phase Fractionation. J Am Soc Mass Spectrom. 26, 1992–2001 (2015)
Soltwisch, J., Goritz, G., Jungmann, J.H., Kiss, A., Smith, D.F., Ellis, S.R., Heeren, R.M.: MALDI mass spectrometry imaging in microscope mode with infrared lasers: bypassing the diffraction limits. Anal. Chem. 86, 321–325 (2014)
Bokhart, M.T., Manni, J., Garrard, K.P., Ekelof, M., Nazari, M., Muddiman, D.C.: IR-MALDESI mass spectrometry imaging at 50 micron spatial resolution. J. Am. Soc. Mass Spectrom. 28(10), 2099–2107 (2017)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Rourke, M.B., Raymond, B.B.A., Djordjevic, S.P. et al. The Effect of Collimating Lens Focusing on Laser Beam Shape in Matrix Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS). J. Am. Soc. Mass Spectrom. 29, 512–515 (2018). https://doi.org/10.1007/s13361-017-1867-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1867-9