Abstract
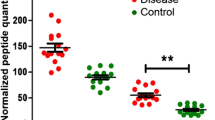
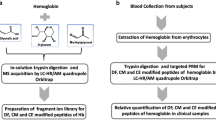
The glycation level at β-Val-1 of the hemoglobin β chain in human blood (HbA1c%) is used to diagnose diabetes and other diseases. However, hemoglobin glycation occurs on multiple sites on different isoforms with different kinetics, but its differential profile has not been clearly demonstrated. In this study, hemoglobin was extracted from the blood of normal and diabetic individuals by protein precipitation. Triplicate solutions prepared from each sample were directly analyzed or digested with multiple enzymes and then analyzed by nano-LC/MS via bottom-up approach for side-by-side characterization. Intact hemoglobin analysis indicated a single glucose-dominant glycation, which showed good correlation with the HbA1c% values. Moreover, full sequence (100 %) of α/β globin was mapped and seven glycation sites were unambiguously assigned. In addition to β-Val-1, two other major sites at α-Lys-61 and β-Lys-66, which contain the common sequence HGKK, and four minor sites (<1 %) on α-Val-1, β-Lys-132, α-Lys-127, and α-Lys-40 were identified. All sites were shown to exhibit similar patterns of site distribution despite different glucose levels. Both the intact mass measurement and bottom-up data consistently indicated that the total glycation percentage of the β-globin was twice higher than the α-globin. Using molecular modeling, the 3D structure of the consensus sequence (HGKK) was shown to contain a phosphate triangle cavity, which helps to catalyze the glycation reaction. For the first time, hemoglobin glycation in normal and diabetic bloods was comparatively characterized in-depth with 100 % sequence coverage. The results provide insight about the HbA1c parameter and help define the new and old markers.

ᅟ





Similar content being viewed by others
References
Miedema, K.: Laboratory tests in diagnosis and management of diabetes mellitus. Practical considerations. Clin. Chem. Lab. Med. 41, 1259–1265 (2003)
Gillery, P.: A history of Hba1c through clinical chemistry and laboratory medicine. Clin. Chem. Lab. Med. 51, 65–74 (2013)
Jeppsson, J.-O., Kobold, U., Barr, J., Finke, A., Hoelzel, W., Hoshino, T.: Approved IFCC reference method for the measurement of HbA1c in human blood. Clin. Chem. Lab. Med. 40, 78–89 (2002)
Shapiro, R., McManus, M.J., Zalut, C., Bunn, H.F.: Sites of nonenzymatic glycosylation of human hemoglobin a. J. Biol. Chem. 255, 3120–3127 (1980)
Shaklai, N., Garlick, R.L., Bunn, H.F.: Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J. Biol. Chem. 259, 3812–3817 (1984)
Higgins, P.J., Bunn, H.F.: Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J. Biol. Chem. 256, 5204–5208 (1981)
Selvin, E., Steffes, M.W., Zhu, H., Matsushita, K., Wagenknecht, L., Pankow, J., Coresh, J., Brancati, F.L.: Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 362, 800–811 (2010)
Sacks, D.B., Nathan, D.M., Lachin, J.M.: Gaps in the glycation gap hypothesis. Clin. Chem. 57, 150–152 (2011)
Cohen, R.M., Smith, E.P.: Frequency of HbA1c discordance in estimating blood glucose control. Curr. Opin. Clin. Nutr. Metab. Care 11, 512–517 (2008)
McCarter, R.J., Hempe, J.M., Gomez, R., Chalew, S.A.: Biological variation in Hba1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 27, 1259–1264 (2004)
Jaisson, S., Gillery, P.: Evaluation of nonenzymatic posttranslational modification-derived products as biomarkers of molecular aging of proteins. Clin. Chem. 56, 1401–1412 (2010)
Nacharaju, P., Acharya, A.S.: Amadori rearrangement potential of hemoglobin at its glycation sites is dependent on the three-dimensional structure of protein. Biochemistry 31, 12673–12679 (1992)
Bai, X., Wang, Z., Huang, C., Wang, Z., Chi, L.: Investigation of non-enzymatic glycosylation of human serum albumin using ion trap-time of flight mass spectrometry. Molecules 17, 8782–8794 (2012)
Peterson, K.P., Pavlovich, J.G., Goldstein, D., Little, R., England, J., Peterson, C.M.: What is hemoglobin a1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin. Chem. 44, 1951–1958 (1998)
Xinyi, Z., Katalin, F.M., John, C., Phillip, D.: Characterization of glycated hemoglobin in diabetic patients: usefulness of electrospray mass spectrometry in monitoring the extent and distribution of glycation. J. Chromatogr. B 759, 1–15 (2001)
Xie, H., Chakraborty, A., Ahn, J., Yu, Y.Q., Dakshinamoorthy, D.P., Gilar, M., Skilton, S.J., Mazzeo, J.R.: Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies. mAbs 2, 379–394 (2010)
Beck, A., Sanglier-Cianférani, S., Van, D.A.: Biosimilar, biobetter, and next generation antibody characterization by mass spectrometry. Anal. Chem. 84, 4637–4646 (2012)
Chen, S.L., Wu, S.L., Huang, L.J., Huang, J.B., Chen, S.H.: A global comparability approach for biosimilar monoclonal antibodies using LC–tandem MS based proteomics. J. Pharm. Biomed. Anal. 80, 126–135 (2013)
Weykamp, C.W., Miedema, K., de Haan, T., Doelman, C.J.A.: Carbamylated hemoglobin interference in glycohemoglobin assays. Clin. Chem. 45, 438–440 (1999)
Prome, D., Blouquit, Y., Ponthus, C., Prome, J.C., Rosa, J.: Structure of the human adult hemoglobin minor fraction a1b by electrospray and secondary ion mass spectrometry. Pyruvic acid as amino-terminal blocking group. J. Biol. Chem. 266, 13050–13054 (1991)
Frolov, A., Hoffmann, P., Hoffmann, R.: Fragmentation behavior of glycated peptides derived from D-glucose, D-fructose, and D-ribose in tandem mass spectrometry. J. Mass Spectrom. 41, 1459–1469 (2006)
Johansen, M.B., Kiemer, L., Brunak, S.: Analysis and prediction of mammalian protein glycation. Glycobiology 16, 844–853 (2006)
Baynes, J.W., Watkins, N.G., Fisher, C.I., Hull, C.J., Patrick, J.S., Ahmed, M.U., Dunn, J.A., Thorpe, S.R.: The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 304, 43–67 (1989)
Shilton, B.H., Walton, D.J.: Sites of glycation of human and horse liver alcohol dehydrogenase in vivo. J. Biol. Chem. 266, 5587–5592 (1991)
Shilton, B.H., Campbell, R.L., Walton, D.J.: Site specificity of glycation of horse liver alcohol dehydrogenase in vitro. Eur. J. Biochem. 215, 567–572 (1993)
Acosta, J., Hettinga, J., Flückiger, R., Krumrei, N., Goldfine, A., Angarita, L., Halperin, J.: Molecular basis for a link between complement and the vascular complications of diabetes. Proc. Natl. Acad. Sci. U. S. A. 97, 5450–5455 (2000)
Shigenori, I., Takashi, N., Daisuke, Y.: Relationship between impaired glycation and the n-terminal structure of the hb görwihl [β5(a2)pro→ala] variant. Hemoglobin 34, 151–156 (2010)
Ito, S., Nakahari, T., Yamamoto, D.: The structural feature surrounding glycated lysine residues in human hemoglobin. Biomed. Res. 32, 217–223 (2011)
Acknowledgment
The authors acknowledge support for this work by the National Science Council in Taiwan (NSC 102-2113-M-006-005-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, SH., Wang, TF., Wu, CH. et al. In-Depth Comparative Characterization of Hemoglobin Glycation in Normal and Diabetic Bloods by LC-MSMS. J. Am. Soc. Mass Spectrom. 25, 758–766 (2014). https://doi.org/10.1007/s13361-014-0830-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-014-0830-2